Glycosaminoglycan
This article may be too technical for most readers to understand. (July 2015) |
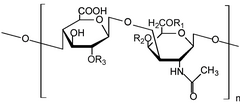
Glycosaminoglycans Because GAGs are highly
Mucopolysaccharidoses are a group of metabolic disorders in which abnormal accumulations of glycosaminoglycans occur due to enzyme deficiencies.
Production
Glycosaminoglycans vary greatly in molecular mass, disaccharide structure, and sulfation. This is because GAG synthesis is not template driven, as are proteins or nucleic acids, but constantly altered by processing enzymes.[5]
GAGs are classified into four groups, based on their core disaccharide structures:[6]
- Heparin/heparan sulfate (HSGAGs)
- rough endoplasmic reticulum, are post-translationally modified via O-linked glycosylation by glycosyltransferases, thus forming proteoglycans.
- Keratan sulfate which may modify core proteins through N-linked glycosylation or O-linked glycosylation of the proteoglycan
- Hyaluronic acid (also known as hyaluronan), which is synthesized by integral membrane synthases, which immediately secrete the dynamically elongated disaccharide chain.[clarification needed]
HSGAG and CSGAG
HSGAG and CSGAG modified proteoglycans first begin with a consensus Ser-Gly/Ala-X-Gly motif in the core protein. Construction of a tetrasaccharide linker that consists of -GlcAβ1–3Galβ1–3Galβ1–4Xylβ1-O-(Ser)-, where xylosyltransferase, β4-galactosyl transferase (GalTI),β3-galactosyl transferase (GalT-II), and β3-GlcA transferase (GlcAT-I) transfer the four monosaccharides, begins synthesis of the GAG modified protein. The first modification of the tetrasaccharide linker determines whether the HSGAGs or CSGAGs will be added. Addition of a GlcNAc promotes the addition of HSGAGs while addition of GalNAc to the tetrasaccharide linker promotes CSGAG development.[6] GlcNAcT-I transfers GlcNAc to the tetrasaccahride linker, which is distinct from glycosyltransferase GlcNAcT-II, the enzyme that is utilized to build HSGAGs. EXTL2 and EXTL3, two genes in the EXT tumor suppressor family, have been shown to have GlcNAcT-I activity. Conversely, GalNAc is transferred to the linker by the enzyme GalNAcT to initiate synthesis of CSGAGs, an enzyme which may or may not have distinct activity compared to the GalNAc transferase activity of chondroitin synthase.[6]
With regard to HSGAGs, a multimeric enzyme encoded by EXT1 and EXT2 of the EXT family of genes, transfers both GlcNAc and GlcA for HSGAG chain elongation. While elongating, the HSGAG is dynamically modified, first by N-deacetylase, N-sulfotransferase (NDST1), which is a bifunctional enzyme that cleaves the N-acetyl group from GlcNAc and subsequently sulfates the N-position. Next, C-5 uronyl epimerase coverts d-GlcA to l-IdoA followed by 2-O sulfation of the uronic acid sugar by 2-O sulfotransferase (Heparan sulfate 2-O-sulfotransferase). Finally, the 6-O and 3-O positions of GlcNAc moities are sulfated by 6-O (Heparan sulfate 6-O-sulfotransferase) and 3-O (3-OST) sulfotransferases.
Chondroitin sulfate and dermatan sulfate, which comprise CSGAGs, are differentiated from each other by the presence of GlcA and IdoA epimers respectively. Similar to the production of HSGAGs, C-5 uronyl epimerase converts d-GlcA to l-IdoA to synthesize dermatan sulfate. Three sulfation events of the CSGAG chains occur: 4-O and/or 6-O sulfation of GalNAc and 2-O sulfation of uronic acid. Four isoforms of the 4-O GalNAc sulfotransferases (C4ST-1, C4ST-2, C4ST-3, and D4ST-1) and three isoforms of the GalNAc 6-O sulfotransferases (C6ST, C6ST-2, and GalNAc4S-6ST) are responsible for the sulfation of GalNAc.[7]
Keratan sulfate types
Unlike HSGAGs and CSGAGs, the third class of GAGs, those belonging to
Hyaluronic acid class

The fourth class of GAG,
Pharmacodynamics
- HSGAGs
- Endogenous heparin is localized and stored in secretory granules of mast cells. Histamine that is present within the granules is protonated (H2A2+) at pH within granules (5.2–6.0), thus it is believed that heparin, which is highly negatively charged, functions to electrostatically retain and store histamine.[13] In the clinic, heparin is administered as an anticoagulant and is also the first line choice for thromboembolic diseases.[14][15] Heparan sulfate (HS) has numerous biological activities and functions, including cell adhesion, regulation of cell growth and proliferation, developmental processes, cell surface binding of lipoprotein lipase and other proteins, angiogenesis, viral invasion, and tumor metastasis.[13]
CSGAGs interact with heparin binding proteins, specifically dermatan sulfate interactions with fibroblast growth factor FGF-2 and FGF-7 have been implicated in cellular proliferation and wound repair
- Keratan sulfates
- One of the main functions of the third class of GAGs, keratan sulfates, is the maintenance of tissue hydration.embryo implantation in the endometrial uterine lining during menstrual cycles, and affect the motility of corneal endothelial cells.[18]In summary, KS plays an anti-adhesive role, which suggests very important functions of KS in cell motility and attachment as well as other potential biological processes.
Dermatan sulfates
Dermatan sulfates function in the skin, tendons, blood vessels, and heart valves.[19]
- Hyaluronic acid
- Hyaluronic acid is a major component of synovial tissues and fluid, as well as the ground substance of other connective tissues. Hyaluronic acid binds cells together, lubricates joints, and helps maintain the shape of the eyeballs.[19]:The viscoelasticity of hyaluronic acid make it ideal for lubricating joints and surfaces that move along each other, such as cartilage. A solution of hyaluronic acid under low shear stress has a much higher viscosity than while under high shear stress.[20] Hyaluronidase, an enzyme produced by white blood cells, sperms cells, and some bacteria, breaks apart the hyaluronic acid, causing the solution to become more liquid.[19]
- In vivo, hyaluronic acid forms randomly kinked coils that entangle to form a hyaluronan network, slowing diffusion and forming a diffusion barrier that regulates transport of substances between cells. For example, hyaluronan helps partition plasma proteins between vascular and extravascular spaces, which affects solubility of macromolecules in the interstitium, changes chemical equilibria, and stabilizes the structure of collagen fibers.[20]
- Other functions include matrix interactions with hyaluronan binding proteins such as hyaluronectin, glial hyaluronan binding protein, brain enriched hyaluronan binding protein, stem cell niche. Stem cells are protected from the effects of growth factors by a shield of hyaluronan and minimally sulfated chondroitin sulfate. During progenitor division, the daughter cell moves outside of this pericellular shield where it can then be influenced by growth factors to differentiate even further.
Classification
Members of the glycosaminoglycan family vary in the type of hexosamine, hexose or hexuronic acid unit they contain (e.g. glucuronic acid, iduronic acid, galactose, galactosamine, glucosamine).
They also vary in the geometry of the glycosidic linkage.
Examples of GAGs include:
| Name | Hexuronic acid or hexose (for keratan) |
Hexosamine | Linkage geometry between predominant monomeric units | Unique features |
|---|---|---|---|---|
| Chondroitin sulfate | GlcUA or GlcUA(2S) |
GalNAc or GalNAc(4S) or GalNAc(6S) or GalNAc(4S,6S) |
GlcUAβ(1→3)GalNAcβ(1→4) | Most prevalent GAG |
| Dermatan sulfate | GlcUA or IdoUA or IdoUA(2S) |
GalNAc or GalNAc(4S) or GalNAc(6S) or GalNAc(4S,6S) |
'IdoUAβ1-3'GalNAcβ1-4 | Distinguished from chondroitin sulfate by the presence of iduronic acid, although some hexuronic acid monosaccharides may be glucuronic acid.[16] |
| Keratan sulfate | Gal or Gal(6S) |
GlcNAc or GlcNAc(6S) |
-Gal(6S)β1-4GlcNAc(6S)β1-3 | Keratan sulfate type II may be fucosylated.[21] |
| Heparin | GlcUA or IdoUA(2S) |
GlcNAc or GlcNS or GlcNAc(6S) or GlcNS(6S) |
-IdoUA(2S)α1-4GlcNS(6S)α1-4 | Highest negative charge density of any known biological molecule |
| Heparan sulfate | GlcUA or IdoUA or IdoUA(2S) |
GlcNAc or GlcNS or GlcNAc(6S) or GlcNS(6S) |
-GlcUAβ1-4GlcNAcα1-4 | Highly similar in structure to heparin, however heparan sulfate's disaccharide units are organised into distinct sulfated and non-sulfated domains.[22] |
| Hyaluronan | GlcUA | GlcNAc | -GlcUAβ1-3GlcNAcβ1-4 | The only GAG that is exclusively non-sulfated |
Abbreviations
- GlcUA = β-D-glucuronic acid
- GlcUA(2S) = 2-O-sulfo-β-D-glucuronic acid
- IdoUA = α-L-iduronic acid
- IdoUA(2S) = 2-O-sulfo-α-L-iduronic acid
- Gal = β-D-galactose
- Gal(6S) = 6-O-sulfo-β-D-galactose
- GalNAc = β-D-N-acetylgalactosamine
- GalNAc(4S) = β-D-N-acetylgalactosamine-4-O-sulfate
- GalNAc(6S) = β-D-N-acetylgalactosamine-6-O-sulfate
- GalNAc(4S,6S) = β-D-N-acetylgalactosamine-4-O, 6-O-sulfate
- GlcNAc = α-D-N-acetylglucosamine
- GlcNS = α-D-N-sulfoglucosamine
- GlcNS(6S) = α-D-N-sulfoglucosamine-6-O-sulfate
See also
References
- ^ "glycosaminoglycan" at Dorland's Medical Dictionary
- ^ "mucopolysaccharide" at Dorland's Medical Dictionary
- ISBN 978-0879695590.
- S2CID 38827411.
- ^ Caligur, Vicki (2008). "Glycosaminoglycan Sulfation and Signaling". Retrieved 25 November 2012.
- ^ PMID 16834555.
- PMID 14568616.
- ^ PMID 12512857.
- PMID 11469798.
- PMID 10617644.
- PMID 8049203.
- PMID 10455188.
- ^ PMID 12137280.
- PMID 9405673.
- ^ Rodén, L. (1989). Lane, DA (ed.). Heparin: Chemical and Biological Properties, Clinical Applications. CRC Press, Inc. p. 1.
- ^ PMID 12213784.
- PMID 14568617.
- ^ PMID 11030741.
- ^ )
- ^ PMID 8724014.
- PMID 11030741.
- ISBN 978-0-8247-0334-9.)
{{cite book}}: CS1 maint: multiple names: authors list (link
External links
- King M. 2005. Glycosaminoglycans. Indiana University School of Medicine Accessed December 31, 2006.
- Glycosaminoglycans at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
- MRI evaluation of glycosaminoglycan loss (dGEMRIC evaluation) Archived 2011-08-22 at the Wayback Machine
