Guide RNA
This article has multiple issues. Please help improve it or discuss these issues on the talk page. (Learn how and when to remove these template messages)
|
Guide RNA (gRNA) or single guide RNA (sgRNA) is a short sequence of RNA that functions as a guide for the Cas9-endonuclease or other Cas-proteins[1] that cut the double-stranded DNA and thereby can be used for gene editing.[2] In bacteria and archaea, gRNAs are a part of the CRISPR-Cas system that serves as an adaptive immune defense that protects the organism from viruses. Here the short gRNAs serve as detectors of foreign DNA and direct the Cas-enzymes that degrades the foreign nucleic acid.[1][3]
History
RNA-editing Guide RNA was discovered in 1990 by B. Blum, N. Bakalara, and L. Simpson in the eukaryotic parasite, Leishmania tarentolaes mitochondrial maxicircle DNA, by Northern Blot Hybridization.[4] Furthermore, several studies have been carried out to determine the structure and function of gRNA and the CRISPR-Cas system in the mid-2000s and the years to follow,[2] with the most notable breakthrough in 2012, when it was discovered that gRNA can be used to guide a Cas9 endonuclease to introduce target specific breaks in double-stranded DNA. This discovery led to a nobels price for Jennifer Doudna and Emmanuelle Charpentier in 2020.[3]
Guide RNA in Protists
The majority of maxicircle transcripts cannot be translated into proteins due to frameshifts in their sequences. These frameshifts are corrected post-transcriptionally through the insertion and deletion of uridine residues at precise sites, which then create an open reading frame. This open reading frame is subsequently translated into a protein that is homologous to mitochondrial proteins found in other cells.[11] The process of uridine insertion and deletion is mediated by short guide RNAs (gRNAs),which encode the editing information through complementary sequences, and allow for base pairing between guanine and uracil (GU) as well as between guanine and cytosine (GC), facilitating the editing process.[12]
The function of the gRNA-mRNA Complex
Guide RNAs are mainly transcribed from the intergenic region of DNA maxicircle and have sequences complementary to mRNA. The 3' end of gRNAs contains an oligo 'U' tail (5-24 nucleotides in length) which is in a nonencoded region but interacts and forms a stable complex with A and G rich regions of pre-edited mRNA and gRNA, that are thermodynamically stabilized by a 5' and 3' anchors.[13] This initial hybrid helps in the recognition of specific mRNA site to be edited.[14]
RNA editing typically progresses from the 3' to the 5' end on the mRNA. The initial editing process begins when a gRNA forms an RNA duplex with a complementary mRNA sequence located just downstream of the editing site. This pairing recruits a number of
In the case of "pan-edited" mRNAs,
It is not clear why trypanosomatids utilize such an elaborate mechanism to produce mRNAs. It might have originated in the early mitochondria of the ancestor of the kintoplastid protist lineage, since it is present in the
Guide RNA sequences
In the protozoan Leishmania tarentolae, 12 of the 18 mitochondrial genes are edited using this process. One such gene is Cyb. The mRNA is actually edited twice in succession. For the first edit, the relevant sequence on the mRNA is as follows:
mRNA 5' AAAGAAAAGGCUUUAACUUCAGGUUGU 3'
The 3' end is used to anchor the gRNA (gCyb-I gRNA in this case) by basepairing (some G/U pairs are used). The 5' end does not exactly match and one of three specific endonucleases cleaves the mRNA at the mismatch site.
gRNA 3' AAUAAUAAAUUUUUAAAUAUAAUAGAAAAUUGAAGUUCAGUA 5' mRNA 5' A A AGAAA A G G C UUUAACUUCAGGUUGU 3'
The mRNA is now "repaired" by adding U's at each editing site in succession, giving the following sequence:
gRNA 3' AAUAAUAAAUUUUUAAAUAUAAUAGAAAAUUGAAGUUCAGUA 5' mRNA 5' UUAUUAUUUAGAAAUUUAUGUUGUCUUUUAACUUCAGGUUGU 3'
This particular gene has two overlapping gRNA editing sites. The 5' end of this section is the 3' anchor for another gRNA (gCyb-II gRNA).[4]
Guide RNA in Prokaryotes
CRISPR In Prokaryotes
Prokaryotes as bacteria and archaea, use CRISPR (clustered regularly interspaced short palindromic repeats) and its associated Cas enzymes, as their adaptive immune system. When prokaryotes are infected by phages, and manage to fend off the attack, specific Cas enzymes cut the phage DNA (or RNA) and integrate the fragments into the CRISPR sequence interspaces. These stored segments are then recognized during future virus attacks, allowing Cas enzymes to use RNA copies of these segments, along with their associated CRISPR sequences, as gRNA to identify and neutralize the foreign sequences.[20][21][22]
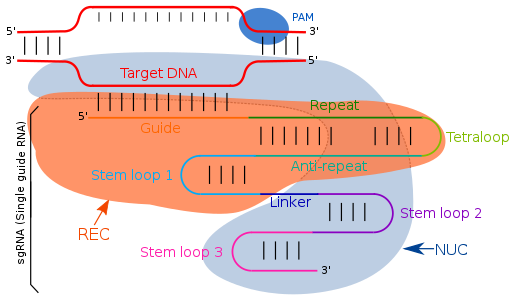
Structure
Guide RNA targets the complementary sequences by simple Watson-Crick base pairing.[23] In the type II CRISPR/cas system, the sgRNA directs the Cas-enzyme to target specific regions in the genome for targeted DNA cleavage. The sgRNA is an artificially engineered combination of two RNA molecules: CRISPR RNA (crRNA) and trans-activating crRNA (tracrRNA). The crRNA component is responsible for binding to the target-specific DNA region, while the tracrRNA component is responsible for the activation of the Cas9 endonuclease activity. These two components are linked by a short tetraloop structure, resulting in the formation of the sgRNA. The tracrRNA consist of base pairs that form a stem-loop structure, enabling its attachment to the endonuclease enzyme. The transcription of the CRISPR locus generates crRNA, which contains spacer regions flanked by repeat sequences, typically 18-20 base pairs (bp) in length. This crRNA guides the Cas9 endonuclease to the complementary target region on the DNA, where it cleaves the DNA, forming what is known as the effector complex. Modifications in the crRNA sequence within the sgRNA can alter the binding location, allowing for precise targeting of different DNA regions, effectively making it a programmable system for genome editing.[24][25][26]
Applications
Designing gRNAs
The targeting specificity of CRISPR-Cas9 is determined by the 20-nucleotide (nt) sequence at the 5' end of the gRNA. The desired target sequence must precede the Protospacer Adjacent Motif (PAM), which is a short DNA sequence usually 2-6 base pairs in length that follows the DNA region targeted for cleavage by the CRISPR system, such as CRISPR-Cas9. The PAM is required for a Cas nuclease to cut and is usually located 3-4 nucleotides downstream from the cut site. Once the gRNA base pairs with the target, Cas9 induces a double-strand break about 3 nucleotides upstream of the PAM.[27][28]
The optimal GC content of the guide sequence should be over 50%. A higher GC content enhances the stability of the RNA-DNA duplex and reduces off-target hybridization. The length of guide sequences is typically 20 bp, but they can also range from 17 to 24 bp. A longer sequence minimizes off-target effects. Guide sequences shorter than 17 bp are at risk of targeting multiple loci.[29][30][24]
CRISPR Cas9
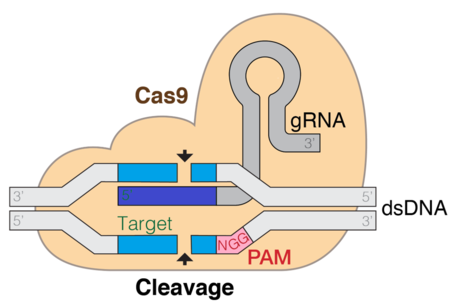
CRISPR (Clustered regularly interspaced short palindromic repeats)/Cas9 is a technique used for gene editing and gene therapy. Cas is an endonuclease enzyme that cuts DNA at a specific location directed by a guide RNA. This is a target-specific technique that can introduce gene knockouts or knock-ins depending on the double strand repair pathway. Evidence shows that both in vitro and in vivo, tracrRNA is required for Cas9 to bind to the target DNA sequence. The CRISPR-Cas9 system consists of three main stages. The first stage involves the extension of bases in the CRISPR locus region by addition of foreign DNA spacers in the genome sequence. Proteins like cas1 and cas2, assist in finding new spacers. The next stage involves transcription of CRISPR: pre-crRNA (precursor CRISPR RNA) are expressed by the transcription of CRISPR repeat-spacer array. Upon further modification, the pre-crRNA is converted to single spacer flanked regions forming short crRNA. RNA maturation process is similar in type I and III but different in type II. The third stage involves binding of cas9 protein and directing it to cleave the DNA segment. The Cas9 protein binds to a combined form of crRNA and tracrRNA forming an effector complex. This serves as guide RNA for the cas9 protein directing its endonuclease activity.[31][2][3]
RNA mutagenesis
One important method of gene regulation is RNA mutagenesis, which can be introduced through RNA editing with the assistance of gRNA.[32] Guide RNA replaces adenosine with inosine at specific target sites, modifying the genetic code.[33] Adenosine deaminase acts on RNA, bringing post transcriptional modification by altering codons and different protein functions. Guide RNAs are small nucleolar RNAs that, along with riboproteins, perform intracellular RNA alterations such as ribomethylation in rRNA and the introduction of pseudouridine in preribosomal RNA.[34] Guide RNAs bind to the antisense RNA sequence and regulate RNA modification. It has been observed that small interfering RNA (siRNA) and micro RNA (miRNA) are generally used as target RNA sequences, and modifications are comparatively easy to introduce due to their small size.[35]
See also
- CRISPR gene editing
- CRISPR/Cas Tools
- SiRNA
- Gene knockout
- Protospacer adjacent motif
References
- ^ PMID 23287722.
- ^ PMID 25430774.
- ^ PMID 22745249.
- ^ S2CID 19656609.
- PMID 12591999.
- PMID 19590014.
- PMID 10580144.
- PMID 17123956.
- ^ PMID 31665448.
- PMID 10668805.
- PMID 1730639.
- ^ PMID 21823228.
- S2CID 2181338.
- ^ PMID 9020135.
- PMID 8978701.
- ^ PMID 20546801.
- PMID 1379519.
- ISBN 978-94-010-3982-6, retrieved 2024-02-24
- PMID 21030427.
- S2CID 205227944.
- PMID 22060043.
- PMID 21531607.
- PMID 15691655.
- ^ PMID 28375731.
- PMID 24728998.
- PMID 23563642.
- PMID 23873081.
- PMID 25184501.
- PMID 24838573.
- PMID 26521937. Retrieved 2024-02-25.
- PMID 23535272.
- PMID 12045112.
- PMID 28148949.
- PMID 2247610.
- PMID 25027649.
Further reading
- Guide RNA-directed uridine insertion RNA editing in vitrohttp://www.jbc.org/content/272/7/4212.full
- Blum, Beat; Simpson, Larry (1990). "Guide RNAs in kinetoplastid mitochondria have a nonencoded 3′ oligo(U) tail involved in recognition of the preedited region". Cell. 62 (2): 391–397. S2CID 2181338.
- Kurata, Morito; Wolf, Natalie K.; Lahr, Walker S.; Weg, Madison T.; Kluesner, Mitchell G.; Lee, Samantha; Hui, Kai; Shiraiwa, Masano; Webber, Beau R.; Moriarity, Branden S. (2018). "Highly multiplexed genome engineering using CRISPR/Cas9 gRNA arrays". PLOS ONE. 13 (9): e0198714. PMID 30222773.
- Khan, Fehad J.; Yuen, Garmen; Luo, Ji (2019). "Multiplexed CRISPR/Cas9 gene knockout with simple crRNA:tracrRNA co-transfection". Cell & Bioscience. 9: 41. PMID 31139343.
- Nishimasu, Hiroshi; Nureki, Osamu (2017). "Structures and mechanisms of CRISPR RNA-guided effector nucleases". Current Opinion in Structural Biology. 43: 68–78. PMID 27912110.
- Chuai, Guohui; Ma, Hanhui; Yan, Jifang; Chen, Ming; Hong, Nanfang; Xue, Dongyu; Zhou, Chi; Zhu, Chenyu; Chen, Ke; Duan, Bin; Gu, Feng; Qu, Sheng; Huang, Deshuang; Wei, Jia; Liu, Qi (2018). "DeepCRISPR: Optimized CRISPR guide RNA design by deep learning". Genome Biology. 19 (1): 80. PMID 29945655.
