Heteropolymetalate

K5[IMo6O24]·nH2O
Ag7[PV12O36]·nH2O
(NH4)4[NiMo6O24H6]·5H2O
K3[CrMo6O24H6]·nH2O
(NH4)8[CeMo12O42]·8H2O
In
Structure
Certain structural motifs recur. The Keggin ion for example is common to both molybdates and tungstates with diverse central heteroatoms. The Keggin and Dawson structures have tetrahedrally-coordinated heteroatoms, such as P or Si, and the Anderson structure[3] has an octahedral central atom, such as aluminium.

|

|

| |
| Strandberg structure, [HP2Mo5O23]4− | Keggin structure, [XM12O40]n− | Dawson structure, [X2M18O62]n− | |

|

|

|

|
| Anderson structure, [XM6O24]n− | Allman–Waugh structure, [XM9O32]n− | Weakley–Yamase structure, [XM10O36]n− | Dexter–Silverton structure, [XM12O42]n− |
Heteropolyacids
Generally, the heteropolymetalates are more thermally robust than homopolymetalates. This trend reflects the stabilizing influence of the tetrahedral oxyanion that "glues" together the transition metal oxo framework. One reflection of their ruggedness, heteropolymetalates can be isolated in their acid form, whereas homopolymetalates typically cannot. Examples include:[4][5]
- Silicotungstic acid, H4SiW12O40·nH2O
- Phosphomolybdic acid, H3Mo12PO40·nH2O
- Phosphotungstic acid, H3W12PO40·nH2O
Isomerism
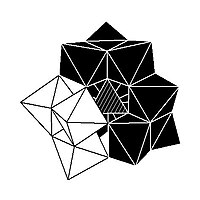
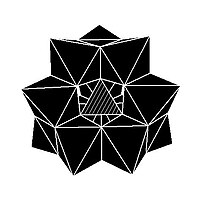
The Keggin structure has 5 isomers, which are obtained by (conceptually) rotating one or more of the four M3O13 units through 60°.[citation needed]
| α-[XM12O40]n− | β-[XM12O40]n− | γ-[XM12O40]n− | δ-[XM12O40]n− | ε-[XM12O40]n− |
|---|---|---|---|---|

|

|

|
|
|
Lacunary structures
The structure of some POMs are derived from a larger POM's structure by removing one or more addenda atoms and their attendant oxide ions, giving a defect structure called a lacunary structure. An example of a compound with a Dawson lacunary structure is As2W15O56.[6] In 2014, vanadate species with similar, selective metal-binding properties were reported.[7]
Uses

This type of acid is a common re-usable acid

The heteropolyacids are widely used as homogeneous and heterogeneous catalysts,[9] particularly those based on the Keggin structure as they can possess qualities such as good thermal stability, high acidity and high oxidising ability. Some examples of catalysis are:[10]
- Homogeneous acid catalysis
- hydrolysis of propan-2-olby H3PMo12O40 and H3PW12O40
- Prins reaction by H3PW12O40
- polymerisation of THF by H3PW12O40
- hydrolysis of
- Heterogeneous acid catalysis
- dehydration of propene and methanolto hydrocarbons by H3PW12O40
- reformation of 2-methylpentane(isohexane) by H3PW12O40 on SiO2
- dehydration of
- Homogeneous oxidation
- cyclohexene + H2O2 to adipic acid by the mixed addenda H3PMo6V6O40
- ketone by O2 to acid and aldehyde by mixed addenda H5PMo10V2O40
Heteropolyacids have long been used in analysis and histology and are a component of many reagents e.g. the
See also
Citations
- ISBN 978-0-7506-3365-9.
- ^ Pope, M. T. (1983). Heteropoly and Isopoly Oxometalates. New York: Springer Verlag.
- .
- ISBN 9781118744994.
- ^ Handbook of Preparative Inorganic Chemistry, 2nd Ed. Edited by G. Brauer, Academic Press, 1963, NY.
- doi:10.1039/b304255c.
- PMID 25082170.
- PMID 11851503.
- PMID 11851502.
- ISBN 0-471-93620-0
References
- ISBN 0-471-19957-5
