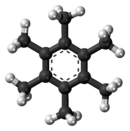
Hexamethylbenzene

| |
| |

| |
| Names | |
|---|---|
| Preferred IUPAC name
Hexamethylbenzene | |
| Other names
1,2,3,4,5,6-Hexamethylbenzene
Mellitene | |
| Identifiers | |
3D model (
JSmol ) |
|
| ChEBI | |
| ChemSpider | |
ECHA InfoCard
|
100.001.616 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C12H18 | |
| Molar mass | 162.276 g·mol−1 |
| Appearance | White crystalline powder |
| Density | 1.0630 g cm−3 |
| Melting point | 165.6 ± 0.7 °C |
| Boiling point | 265.2 °C (509.4 °F; 538.3 K) |
| insoluble | |
| Solubility | acetic acid, acetone, benzene, chloroform, diethyl ether, ethanol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Hexamethylbenzene, also known as mellitene, is a
Hexamethylbenzene can be oxidised to
In 2016 the crystal structure of the hexamethylbenzene
6(CH
3)2+
6 was first observed.[15][16][17]
Nomenclature and properties
According to the
Hexamethylbenzene is sometimes called mellitene,

Treatment of hexamethylbenzene with a superelectrophilic mixture of methyl chloride and aluminum trichloride (a source of Meδ⊕Cl---δ⊖AlCl3) gives heptamethylbenzenium cation, one of the first carbocations to be directly observed.
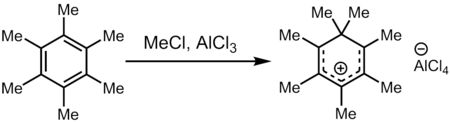
Structure
In 1927
Preparation
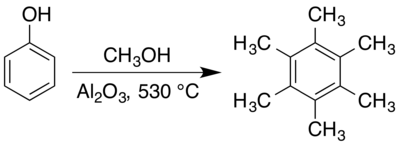
The compound can be prepared by reacting
In 1880, Joseph Achille Le Bel and William H. Greene reported[36] what has been described as an "extraordinary" zinc chloride-catalysed one-pot synthesis of hexamethylbenzene from methanol.[37] At the catalyst's melting point (283 °C), the reaction has a Gibbs free energy (ΔG) of −1090 kJ mol−1 and can be idealised as:[37]
- 15 CH
3OH → C
6(CH
3)
6 + 3 CH
4 + 15 H
2O
Le Bel and Greene rationalised the process as involving
Hexamethylbenzene is typically prepared in the
The mechanisms of such surface-mediated reactions have been investigated, with an eye to achieving greater control over the outcome of the reaction,

Uses
Synthetic uses
Hexamethylbenzene can be used as a
Other uses
Hexamethylbenzene has no commercial or widespread uses. It is exclusively of interest for chemical research.
Reactions
It forms orange-yellow 1:1 adduct with
Oxidation with trifluoroperacetic acid or hydrogen peroxide gives 2,3,4,5,6,6-hexamethyl-2,4-cyclohexadienone:[45][27][32])

It has also been used as a solvent for 3He-NMR spectroscopy.[46]
Just as with benzene itself, the electron-rich aromatic system in hexamethylbenzene allows it to act as a
6(CH
3)
6)
2]n+ (M = Co, Fe, Rh, Ru; n = 1, 2), where the metal centre is bound by the π electrons of the two arene moieties, and can easily be synthesised from appropriate metal salts by ligand exchange, for example:[48]
- CoBr
2 + 2 AlBr
3 → [Co(C
6(CH
3)
6)
2]2+
+ 2 AlBr−
4
The complexes can undergo redox reactions. The rhodium and cobalt dications undergo a one-electron reduction with a suitable active metal (aluminium for the cobalt system, zinc for the rhodium), and the equations describing the reactions in the cobalt system are as follows:[48]
- 3 [Co(C
6(CH
3)
6)
2]2+
+ Al → 3 [Co(C
6(CH
3)
6)
2]+
+ Al3+

Left: n = 2, [RuII(η6-C6(CH3)6)2]2+
Right: n = 0, [Ru0(η4-C6(CH3)6)(η6-C6(CH3)6)]
Methyl groups omitted for clarity. The electron-pairs involved with carbon–ruthenium bonding are in red.
In the field of organoruthenium chemistry, the redox interconversion of the analogous two-electron reduction of the dication and its neutral product occurs at −1.02 V in acetonitrile[7] and is accompanied by a structural change.[8][50] The hapticity of one of the hexamethylbenzene ligands changes with the oxidation state of the ruthenium centre, the dication [Ru(η6-C6(CH3)6)2]2+ being reduced to [Ru(η4-C6(CH3)6)(η6-C6(CH3)6)],[8] with the structural change allowing each complex to comply with the 18-electron rule and maximise stability.
The equivalent iron(II) complex undergoes a reversible one-electron reduction (at −0.48 V in aqueous ethanol), but the two-electron reduction (at −1.46 V) is irreversible,[7] suggesting a change in structure different from that found in the ruthenium system.
Dication

6(CH
3)2+
6
The isolation of an ion with composition C
6(CH
3)
6H+
was first reported from investigations of
6(CH
3)2+
6, and the suggestion was soon supported by experimental evidence.[15][16][17] Spectroscopic investigation of the two-electron oxidation of benzene at very low temperatures (below 4 K) shows that a hexagonal dication forms and then rapidly rearranges into a pyramidal structure:[54]
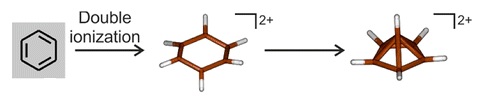

6(CH
3)2+
6 having a rearranged pentagonal-pyramid framework
Two-electron oxidation of hexamethylbenzene would be expected to result in a near-identical rearrangement to a
) to form the dication:[9]

Though indirect spectroscopic evidence and theoretical calculations previously pointed to their existence, the isolation and structural determination of a species with a hexacoordinate carbon bound only to other carbon atoms is unprecedented,[9] and has attracted comment in Chemical & Engineering News,[11] New Scientist,[10] Science News,[12] and ZME Science.[55] The carbon atom at the top of the pyramid is bonding with six other atoms, an unusual arrangement as the usual maximum valence for this element is four.[11] The molecule is aromatic and avoids exceeding the octet on carbon by having only a total of six electrons in the five bonds between the base of the pyramid and its apex. That is, each of the vertical edges of the pyramid is only a partial bond rather than a normal covalent bond that would have two electrons shared between two atoms. Although the top carbon does bond to six others, it does so using a total of no more than eight electrons.[14]
The dication, noting the weak bonds forming the upright edges of the pyramid, shown as dashed lines in the structure, have a Wiberg
6(CH
3)2+
6, as drawn by Steven Bachrach[13]
Right: The analogous organometallic complex [(η5
–C
5(CH
3)
5)Zn(CH
3)][56]
It has been commented that "[i]t's super important that people realize that, although we're taught carbon can only have four friends, carbon can be associated with more than four atoms" and added that the "carbon isn't making six bonds in the sense that we usually think of a carbon-carbon bond as a two-electron bond."[12] "It is all about the challenge and the possibility to astonish chemists about what can be possible."[10]
References
- ^ .
- ^ a b Lydon, John (January 2006). "A Welcome to Leeds" (PDF). Newsletter of the History of Physics Group (19): 8–11.
- ^ a b c d Lydon, John (July 2006). "Letters" (PDF). Newsletter of the History of Physics Group (20): 34–35.
- ^ .
- ^ ISBN 9781316423684.
- ^ .
- ^ ISBN 9781489920348.
- ^ .
- ^ PMID 27885766.
- ^ a b c d Boyle, Rebecca (14 January 2017). "Carbon seen bonding with six other atoms for the first time". New Scientist (3108). Archived from the original on 16 January 2017. Retrieved 14 January 2017.
- ^ from the original on 9 January 2017.
- ^ a b c d Hamers, Laurel (24 December 2016). "Carbon can exceed four-bond limit". Science News. 190 (13): 17. Archived from the original on 3 February 2017.
- ^ a b c d Bachrach, Steven M. (17 January 2017). "A six-coordinate carbon atom". comporgchem.com. Archived from the original on 19 January 2017. Retrieved 18 January 2017.
- ^ .
- ^ .
- ^ .
- ^ .
- ISBN 9780854041824.
- ^ ISBN 9781439880500.
- ^ ; Collected Volumes, vol. 4, p. 520.
- Perseus Digital Library, Tufts University..
- .
- S2CID 4105837.
- ^ Lonsdale, Kathleen (1948). Crystals and X-Rays. George Bell & Sons.
- ^ .
- ^ .
- ^ .
- ^ .
- ^ .
- ^ .
- ^ PMID 21008356.
- ^ .
- ^ hdl:2027.42/22975.
- ^ .
- ^ .
- ^ Le Bel, Joseph Achille; Greene, William H. (1880). "On the decomposition of alcohols, etc., by zinc chloride at high temperatures". American Chemical Journal. 2: 20–26.
- ^ .
- .
- ; Collected Volumes, vol. 2, p. 248.
- .
- .
- .
- .
- .
- ; Collected Volumes, vol. 5, p. 598.
- .
- ISBN 9780471238966.
- ^ .
- .
- )
- .
- .
- .
- PMID 24528384.
- ^ Puiu, Tibi (5 January 2017). "Exotic carbon molecule has six bonds, breaking the four-bond limit". zmescience.com. ZME Science. Archived from the original on 16 January 2017. Retrieved 14 January 2017.
- ^ .
- ISBN 9783527805143.




