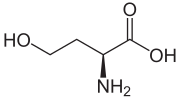
Homoserine
| |

| |
| Names | |
|---|---|
| IUPAC name
(S)-2-Amino-4-hydroxybutanoic acid
| |
| Identifiers | |
3D model (
JSmol ) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
ECHA InfoCard
|
100.010.538 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C4H9NO3 | |
| Molar mass | 119.12 g/mol |
| Melting point | 203 °C (decomposes) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Homoserine (also called isothreonine) is an α-amino acid with the chemical formula HO2CCH(NH2)CH2CH2OH. L-Homoserine is not one of the common amino acids encoded by DNA. It differs from the proteinogenic amino acid serine by insertion of an additional -CH2- unit into the backbone. Homoserine, or its lactone form, is the product of a cyanogen bromide cleavage of a peptide by degradation of methionine.
Homoserine is an intermediate in the
Applications
Commercially, homoserine can serve as precursor to the synthesis of isobutanol and 1,4-butanediol.[4] Purified homoserine is used in enzyme structural studies.[5] Also, homoserine has played important roles in studies to elucidate peptide synthesis and synthesis of proteoglycan glycopeptides.[6] Bacterial cell lines can make copious amounts of this amino acid.[3][4]
Biosynthesis
Homoserine is produced from aspartate via aspartate-4-semialdehyde, which is produced from β-phosphoaspartate. By the action of homoserine dehydrogenases, the semialdehyde is converted to homoserine.[7]

L-Homoserine is substrate for homoserine kinase, yielding phosphohomoserine (homoserine-phosphate), which is converted to by threonine synthase to yield L-threonine.
Homoserine is converted to O-succinyl homoserine by homoserine O-succinyltransferase, a precursor to L-methionine.[8]
Homoserine allosterically inhibits aspartate kinase and
References
