Hydroboration

In
.Hydroboration produces
The development of this technology and the underlying concepts were recognized by the Nobel Prize in Chemistry to Herbert C. Brown.[1] He shared the prize with Georg Wittig in 1979[2] for his pioneering research on organoboranes as important synthetic intermediates. A complement to hydroboration is carboboration, where a carbon moiety is incorporated rather than hydrogen.
Addition of a H-B bond to C-C double bonds
Hydroboration is typically anti-Markovnikov, i.e. the hydrogen adds to the most substituted carbon of the double bond. That the regiochemistry is reverse of a typical HX addition reflects the polarity of the Bδ+-Hδ− bonds. Hydroboration proceeds via a four-membered transition state: the hydrogen and the boron atoms added on the same face of the double bond. Granted that the mechanism is concerted, the formation of the C-B bond proceeds slightly faster than the formation of the C-H bond. As a result, in the transition state, boron develops a partially negative charge while the more substituted carbon bears a partially positive charge. This partial positive charge is better supported by the more substituted carbon. Formally, the reaction is an example of a group transfer reaction. However, an analysis of the orbitals involved reveals that the reaction is 'pseudopericyclic' and not subject to the Woodward–Hoffmann rules for pericyclic reactivity.
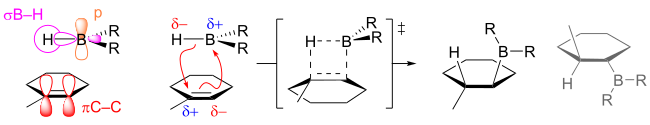
If BH3 is used as the hydroborating reagent, reactions typically proceed beyond the monoalkyl borane compounds, especially for less sterically hindered small olefins. Trisubstituted olefins can rapidly produce dialkyl boranes, but further alkylation of the organoboranes is slowed because of steric hindrance. This significant rate difference in producing di- and tri-alkyl boranes is useful in the synthesis of bulky boranes that can enhance regioselectivity.

Reactions involving substituted alkenes
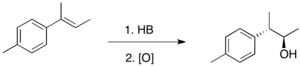
For trisubstituted alkenes such as 1, boron is predominantly placed on the less substituted carbon.[3] The minor product, in which the boron atom is placed on the more substituted carbon, is usually produced in less than 10%. A notable case with lower regioselectivity is styrene, and the selectivity is strongly influenced by the substituent on the para position.

Hydroboration of 1,2-disubstituted alkenes, such as a cis or trans olefin, produces generally a mixture of the two organoboranes of comparable amounts, even if the substituents are very different in terms of steric bulk. For such 1,2-disubstituted olefins, regioselectivity can be observed only when one of the two substituents is a phenyl ring. In such cases, such as trans-1-phenylpropene, the boron atom is placed on the carbon adjacent to the phenyl ring. The observations above indicate that the addition of H-B bond to olefins is under electronic control rather than steric control.


Reactions of organoboranes
The C-B bonds generated by hydroboration are reactive with various reagents, the most common one being
Hydroboration can also lead to amines by treating the intermediate organoboranes with monochloramine or O-hydroxylaminesulfonic acid (HSA).[5]
Terminal olefins are converted to the corresponding
Borane adducts

Diborane can be produced
The adduct BH3(THF) is also commercially available as THF solutions wherein it exists as the 1:1 adduct. It degrades with time.[11]
Borane adducts with phosphines and amines are also available, but are not widely used.[12] Borane makes a strong adduct with triethylamine; using this adduct requires harsher conditions in hydroboration. This can be advantageous for cases such as hydroborating trienes to avoid polymerization. More sterically hindered tertiary and silyl amines can deliver borane to alkenes at room temperature.

Monosubstituted boranes
Monoalkyl boranes are relatively rare. When the alkyl group is small, such as methyl, the monoalkylboranes tend to
- B2H6 + 2 Me2C=CMe2 → [Me2CHCMe2BH2]2
A chiral example is monoisopinocampheylborane. Although often written as IpcBH2, it is a dimer [IpcBH2]2. It is obtained by hydroboration of (−)‐α‐pinene with
Species of the form RBH2 are available for R =
Disubstituted boranes
Dimesitylborane
dimesitylborane is a dimer (C6H2Me3)2B2H2). It reacts only slowly with simple terminal alkenes. On the other hand, alkynes undergo monohydroboration with Mes2BH easily to produce alkenylboranes.[16]
Disiamylborane
Among hindered dialkylboranes is disiamylborane, abbreviated Sia2BH. It also is a dimer. Owing to its steric bulk, it selectively hydroborates less hindered, usually terminal alkenes in the presence of more substituted alkenes.[17] Disiamylborane must be freshly prepared as its solutions can only be stored at 0 °C for a few hours. Dicyclohexylborane Chx2BH exhibits improved thermal stability than Sia2BH.
9-BBN
A versatile dialkylborane is
Other secondary boranes
Simple, unhindered dialkylboranes are reactive at room temperature towards most alkenes and terminal alkynes but are difficult to prepare in high purity, since they exist in equilibrium with mono- and trialkylboranes. One common way of preparing them is the reduction of dialkylhalogenoboranes with metal hydrides.[19] An important synthetic application using such dialkylboranes, such as diethylborane, is the transmetallation of the organoboron compounds to form organozinc compounds.[20][21]
Pinacolborane and catecholborane
For catalytic hydroboration, pinacolborane and catecholborane are widely used. They also exhibit higher reactivity toward alkynes.[22] Pinacolborane is also widely used in a catalyst-free hydroborations.
See also
References
- .
- ^ "The Nobel Prize in Chemistry 1979". www.nobelprize.org. Retrieved 21 March 2017.
- .
- .
- ^ Hydroxylamine
- .
- .
- .
- ^ See Borane-dimethylsulfide complex
- ISBN 0471936235.
- .
- .
- .
- ISBN 9780470842898.
- PMID 11485456.
- .
- .
- ^ Dhillon, R. S. (2007). Hydroboration and Organic Synthesis : 9-Borabicyclo [3.3.1] Nonane (9-BBN). Springer.
- .
- PMID 10941068.
- PMID 11429854.
- ISBN 978-0-9708441-0-1.
