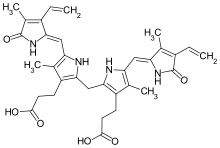
Bilirubin
| |

| |
| Names | |
|---|---|
| IUPAC name
3,3′-(2,17-Diethenyl-3,7,13,18-tetramethyl-1,19-dioxo-10,19,21,22,23,24-hexahydro-1H-biline-8,12-diyl)dipropanoic acid
| |
| Systematic IUPAC name
3,3′-([12(2)Z,6(72)Z]-13,74-Diethenyl-14,33,54,73-tetramethyl-15,75-dioxo-11,15,71,75-tetrahydro-31H,51H-1,7(2),3,5(2,5)-tetrapyrrolaheptaphane-12(2),6(72)-diene-34,53-diyl)dipropanoic acid | |
| Other names
Bilirubin IXα
| |
| Identifiers | |
3D model (
JSmol ) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
ECHA InfoCard
|
100.010.218 |
IUPHAR/BPS |
|
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C33H36N4O6 | |
| Molar mass | 584.673 g·mol−1 |
| Density | 1.31 g·cm-3[1] |
| Melting point | 235°C[2] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Bilirubin (BR) (from the Latin for "red bile") is a red-orange compound that occurs in the normal catabolic pathway that breaks down heme in vertebrates. This catabolism is a necessary process in the body's clearance of waste products that arise from the destruction of aged or abnormal red blood cells.[3] In the first step of bilirubin synthesis, the heme molecule is stripped from the hemoglobin molecule. Heme then passes through various processes of porphyrin catabolism, which varies according to the region of the body in which the breakdown occurs. For example, the molecules excreted in the urine differ from those in the feces.[4] The production of biliverdin from heme is the first major step in the catabolic pathway, after which the enzyme biliverdin reductase performs the second step, producing bilirubin from biliverdin.[5][6]
Ultimately, bilirubin is broken down within the body, and its metabolites excreted through bile and urine; elevated levels may indicate certain diseases.[7] It is responsible for the yellow color of healing bruises and the yellow discoloration in jaundice. The bacterial enzyme bilirubin reductase is responsible for the breakdown of bilirubin in the gut.[8] One breakdown product, urobilin, is the main component of the straw-yellow color in urine.[9] Another breakdown product, stercobilin, causes the brown color of feces.
Although bilirubin is usually found in animals rather than plants, at least one plant species, Strelitzia nicolai, is known to contain the pigment.[10]
Structure
Bilirubin consists of an open-chain tetrapyrrole. It is formed by oxidative cleavage of a porphyrin in heme, which affords biliverdin. Biliverdin is reduced to bilirubin. After conjugation with glucuronic acid, bilirubin is water-soluble and can be excreted.[11]
Bilirubin is structurally similar to the pigment phycobilin used by certain algae to capture light energy, and to the pigment phytochrome used by plants to sense light. All of these contain an open chain of four pyrrolic rings.[citation needed]
Like these other pigments, some of the double-bonds in bilirubin
Some textbooks and research articles show the incorrect geometric isomer of bilirubin.[13] The naturally occurring isomer is the Z,Z-isomer.
Function
Bilirubin is created by the activity of biliverdin reductase on biliverdin, a green tetrapyrrolic bile pigment that is also a product of heme catabolism. Bilirubin, when oxidized, reverts to become biliverdin once again. This cycle, in addition to the demonstration of the potent antioxidant activity of bilirubin,[14] has led to the hypothesis that bilirubin's main physiologic role is as a cellular antioxidant.[15][16] Consistent with this, animal studies suggest that eliminating bilirubin results in endogenous oxidative stress.[17] Bilirubin's antioxidant activity may be particularly important in the brain, where it prevents excitotoxicity and neuronal death by scavenging superoxide during N-methyl-D-aspartic acid neurotransmission.[18]
Metabolism


Bilirubin in plasma is mostly produced by the destruction of erythrocytes. Heme is metabolized into biliverdin (via heme oxygenase) and then into bilirubin (via biliverdin reductase) inside the macrophages. [11]
Bilirubin is then released into the plasma and transported to the liver bound by albumin, since it is insoluble in water in this state. In this state, bilirubin is called unconjugated (despite being bound by albumin). [11]
In the liver, unconjugated bilirubin is up-taken by the hepatocytes and subsequently conjugated with glucuronic acid (via the enzyme
Conjugated bilirubin is excreted into the bile ducts and enters the duodenum. During its transport to the colon, it is converted into urobilinogen by the bacterial enzyme bilirubin reductase.[8] Most of the urobilinogen is further reduced into stercobilinogen and is excreted through feces (air oxidizes stercobilinogen to stercobilin, which gives feces their characteristic brown color). [11]
A lesser amount of urobilinogen is re-absorbed into portal circulation and transferred to the liver. For the most part, this urobilinogen is recycled to conjugated bilirubin and this process closes the enterohepatic circle. There is also an amount of urobilinogen which is not recycled, but rather enters the systemic circulation and subsequently the kidneys, where it is excreted. Air oxidizes urobilinogen into urobilin, which gives urine its characteristic color.[11][19]
In parallel, a small amount of conjugated billirubin can also enter the systemic circulation and get excreted through urine. This is exaggerated in various pathological situations.[19]
Toxicity
Hyperbilirubinemia
Hyperbilirubinemia is a higher-than-normal level of bilirubin in the blood. Hyperbilirubinemia may refer to increased levels of conjugated, unconjugated or both conjugated and unconjugated bilirubin. The causes of hyperbilirubinemia can also be classified into prehepatic, intrahepatic, and posthepatic. [20]
Prehepatic causes are associated mostly with an increase of unconjugated (indirect) bilirubin.[20] They include:
- Hemolysis or increased breakdown of red blood cells (for example hematoma resorption)
Intrahepatic causes can be associated with elevated levels of conjugated bilirubin, unconjugated bilirubin or both.[20] They include:[20]
- Neonatal hyperbilirubinemia, where the newborn's liver is not able to properly process the bilirubin causing jaundice
- Hepatocellular disease
- Viral infections (hepatitis A, B, and C)
- Chronic alcohol use
- Autoimmune disorders
- Genetic syndromes:
- Gilbert's syndrome – a genetic disorder of bilirubin metabolism that can result in mild jaundice, found in about 5% of the population
- Rotor syndrome: non-itching jaundice, with rise of bilirubin in the patient's serum, mainly of the conjugated type
- Dubin–Johnson syndrome
- Crigler–Najjar syndrome
- Pharmaceutical drugs (especially antipsychotic, some sex hormones, and a wide range of other drugs)
- Sulfonamides are contraindicated in infants less than 2 months old (exception when used with pyrimethamine in treating toxoplasmosis) as they increase unconjugated bilirubin leading to kernicterus.[21]
- Drugs such as UGT1A1 enzyme.[22]
Post-hepatic causes are associated with elevated levels of conjugated bilirubin. [20] These include:[20]
- Unusually large bile duct obstruction, e.g. gallstone in common bile duct (which is the most common post-hepatic cause)
- Biliary stricture (benign or malignant)
- Cholangitis
- Severe liver failure with primary biliary cirrhosis)
- Pancreatitis
Cirrhosis may cause normal, moderately high or high levels of bilirubin, depending on exact features of the cirrhosis.
To further elucidate the causes of jaundice or increased bilirubin, it is usually simpler to look at other
Jaundice
Hemoglobin acts to transport oxygen which the body receives to all body tissue via blood vessels. Over time, when red blood cells need to be replenished, the hemoglobin is broken down in the spleen; it breaks down into two parts: heme group consisting of iron and bile and protein fraction. While protein and iron are utilized to renew red blood cells, pigments that make up the red color in blood are deposited into the bile to form bilirubin.[23] Jaundice leads to raised bilirubin levels that in turn negatively remove elastin-rich tissues.[24] Jaundice may be noticeable in the sclera of the eyes at levels of about 2 to 3 mg/dl (34 to 51 μmol/L),[25] and in the skin at higher levels.[note 1]
Jaundice is classified, depending upon whether the bilirubin is free or conjugated to glucuronic acid, into conjugated jaundice or unconjugated jaundice.[citation needed]
Kernicterus
Unbound bilirubin (Bf) levels can be used to predict the risk of neurodevelopmental handicaps within infants.
Health benefits
In the absence of liver disease, high levels of total bilirubin confers various health benefits.[28] Studies have also revealed that levels of serum bilirubin (SBR)[29] are inversely related to risk of certain heart diseases.[30][31] While the poor solubility and potential toxicity of bilirubin limit its potential medicinal applications, current research is being done on whether bilirubin encapsulated silk fibrin nanoparticles can alleviate symptoms of disorders such as acute pancreatitis.[32] In addition to this, there has been recent discoveries linking bilirubin and its ε-polylysine-bilirubin conjugate (PLL-BR), to more efficient insulin medication. It seems that bilirubin exhibits protective properties during the islet transplantation process when drugs are delivered throughout the bloodstream.[33]
Blood tests
Bilirubin is degraded by light. Blood collection tubes containing blood or (especially) serum to be used in bilirubin assays should be protected from illumination.[34] For adults, blood is typically collected by needle from a vein in the arm.[35] In newborns, blood is often collected from a heel stick, a technique that uses a small, sharp blade to cut the skin on the infant's heel and collect a few drops of blood into a small tube. Non-invasive technology is available in some health care facilities that will measure bilirubin by using an bilirubinometer which shines light onto the skin and calculates the amount of bilirubin by analysing how the light is absorbed or reflects. [36] This device is also known as a transcutaneous bilirubin meter.[37]
Bilirubin (in blood) is found in two forms:
| Abb. | Name(s) | Water-soluble | Reaction |
| "BC" | "Conjugated bilirubin" | Yes (bound to glucuronic acid) | Reacts quickly when dyes (diazo reagent) are added to the blood specimen to produce azobilirubin "Direct bilirubin" |
| "BU" | "Unconjugated bilirubin" | No | Reacts more slowly, still produces azobilirubin, Ethanol makes all bilirubin react promptly, then: indirect bilirubin = total bilirubin – direct bilirubin |
Note: Conjugated bilirubin is often incorrectly called "direct bilirubin" and unconjugated bilirubin is incorrectly called "indirect bilirubin". Direct and indirect refer solely to how compounds are measured or detected in solution. Direct bilirubin is any form of bilirubin which is water-soluble and is available in solution to react with assay reagents; direct bilirubin is often made up largely of conjugated bilirubin, but some unconjugated bilirubin (up to 25%) can still be part of the "direct" bilirubin fraction. Likewise, not all conjugated bilirubin is readily available in solution for reaction or detection (for example, if it is hydrogen bonding with itself) and therefore would not be included in the direct bilirubin fraction.[citation needed]
Total bilirubin (TBIL) measures both BU and BC. Total bilirubin assays work by using surfactants and accelerators (like caffeine) to bring all of the different bilirubin forms into solution where they can react with assay reagents. Total and direct bilirubin levels can be measured from the blood, but indirect bilirubin is calculated from the total and direct bilirubin.
Indirect bilirubin is fat-soluble and direct bilirubin is water-soluble.[38]
Total bilirubin
Total bilirubin = direct bilirubin + indirect bilirubin[39]
Elevation of both
Indirect (unconjugated)
The measurement of unconjugated bilirubin (UCB) is underestimated by measurement of indirect bilirubin, as unconjugated bilirubin (without/yet glucuronidation) reacts with diazosulfanilic acid to create azobilirubin which is measured as direct bilirubin.[41][42]
Direct
Direct bilirubin = Conjugated bilirubin + delta bilirubin[39]
Conjugated
In the liver, bilirubin is conjugated with
There, colonic bacteria deconjugate and metabolize the bilirubin into colorless urobilinogen, which can be oxidized to form urobilin and stercobilin. Urobilin is excreted by the kidneys to give urine its yellow color and stercobilin is excreted in the feces giving stool its characteristic brown color. A trace (~1%) of the urobilinogen is reabsorbed into the enterohepatic circulation to be re-excreted in the bile.[44]
Conjugated bilirubin's half-life is shorter than delta bilirubin.[45]
Delta bilirubin
Although the terms direct and indirect bilirubin are used equivalently with conjugated and unconjugated bilirubin, this is not quantitatively correct, because the direct fraction includes both conjugated bilirubin and δ bilirubin.[citation needed]
Delta bilirubin is albumin-bound conjugated bilirubin.
δ bilirubin = total bilirubin – (unconjugated bilirubin + conjugated bilirubin)[39]
Half-life
The half-life of delta bilirubin is equivalent to that of albumin since the former is bound to the latter, yields 2–3 weeks.[47][41]
A free-of-bound bilirubin has a half-life of 2 to 4 hours.[47]
Measurement methods
Originally, the Van den Bergh reaction was used for a qualitative estimate of bilirubin.
This test is performed routinely in most medical laboratories and can be measured by a variety of methods.[48]
Total bilirubin is now often measured by the 2,5-dichlorophenyldiazonium (DPD) method, and direct bilirubin is often measured by the method of Jendrassik and Grof.[49]
Blood levels
The bilirubin level found in the body reflects the balance between production and excretion. Blood test results are advised to always be interpreted using the reference range provided by the laboratory that performed the test. The
- 0–0.3 mg/dl – Direct (conjugated) bilirubin level
- 0.1–1.2 mg/dl – Total serum bilirubin level
| μmol/l = micromole/litre | mg/dl = milligram/ decilitre | |
| total bilirubin | <21[52] | <1.23 |
| direct bilirubin | 1.0–5.1[53] | 0–0.3,[54] 0.1–0.3,[53] 0.1–0.4[55] |
Urine tests
Urine bilirubin may also be clinically significant.[57] Bilirubin is not normally detectable in the urine of healthy people. If the blood level of conjugated bilirubin becomes elevated, e.g. due to liver disease, excess conjugated bilirubin is excreted in the urine, indicating a pathological process.[58] Unconjugated bilirubin is not water-soluble and so is not excreted in the urine. Testing urine for both bilirubin and urobilinogen can help differentiate obstructive liver disease from other causes of jaundice.[59]
As with billirubin, under normal circumstances, only a very small amount of urobilinogen is excreted in the urine. If the liver's function is impaired or when biliary drainage is blocked, some of the conjugated bilirubin leaks out of the hepatocytes and appears in the urine, turning it dark amber. However, in disorders involving hemolytic anemia, an increased number of red blood cells are broken down, causing an increase in the amount of unconjugated bilirubin in the blood. Because the unconjugated bilirubin is not water-soluble, one will not see an increase in bilirubin in the urine. Because there is no problem with the liver or bile systems, this excess unconjugated bilirubin will go through all of the normal processing mechanisms that occur (e.g., conjugation, excretion in bile, metabolism to urobilinogen, reabsorption) and will show up as an increase of urobilinogen in the urine. This difference between increased urine bilirubin and increased urine urobilinogen helps to distinguish between various disorders in those systems.[59]
History
In ancient history, Hippocrates discussed bile pigments in two of the four humours in the context of a relationship between yellow and black biles.[60] Hippocrates visited Democritus in Abdera who was regarded as the expert in melancholy "black bile".[60]
Relevant documentation emerged in 1827 when
Leopold Gmelin experimented with nitric acid in 1826 to establish the redox behavior in change from bilirubin to biliverdin, although the nomenclature did not exist at the time.[60] The term biliverdin was coined by Jöns Jacob Berzelius in 1840, although he preferred "bilifulvin" (yellow/red) over "bilirubin" (red). The term "bilirubin" was thought to have become mainstream based on the works of Staedeler in 1864 who crystallized bilirubin from cattle gallstones.[60][61]
Rudolf Virchow in 1847 recognized hematoidin to be identical to bilirubin.[62] It is not always distinguished from hematoidin, which one modern dictionary defines as synonymous with it[63] but another defines as "apparently chemically identical with bilirubin but with a different site of origin, formed locally in the tissues from hemoglobin, particularly under conditions of reduced oxygen tension."[64][60] The synonymous identity of bilirubin and hematoidin was confirmed in 1923 by Fischer and Steinmetz using analytical crystallography.[60]
In the 1930s, significant advances in bilirubin isolation and synthesis were described by Hans Fischer, Plieninger, and others,[60] and pioneering work pertaining to endogenous formation of bilirubin from heme was likewise conducted in the same decade.[65] The suffix IXα is partially based on a system developed Fischer, which means the bilin's parent compound was protoporphyrin IX cleaved at the alpha-methine bridge (see protoporphyrin IX nomenclature).[66]
Origins pertaining to the physiological activity of bilirubin were described by Ernst Stadelmann in 1891, who may have observed the biotransformation of infused hemoglobin into bilirubin possibly inspired by Ivan Tarkhanov's 1874 works.[60] Georg Barkan suggested the source of endogenous bilirubin to be from hemoglobin in 1932.[67] Plieninger and Fischer demonstrated an enzymatic oxidative loss of the alpha-methine bridge of heme resulting in a bis-lactam structure in 1942.[60] It is widely accepted that Irving London was the first to demonstrate endogenous formation of bilirubin from hemoglobin in 1950,[68] and Sjostrand demonstrated hemoglobin catabolism produces carbon monoxide between 1949 and 1952.[65] 14C labeled protoporphyrin biotransformation to bilirubin evidence emerged in 1966 by Cecil Watson.[60] Rudi Schmid and Tenhunen discovered heme oxygenase, the enzyme responsible, in 1968.[65] Earlier in 1963, Nakajima described a soluble "heme alpha-methnyl oxygeanse" which what later determined to be a non-enzymatic pathway, such as formation of a 1,2-Dioxetane intermediate at the methine bridge resulting in carbon monoxide release and biliverdin formation.[66]
Notable people
- Claudio Tiribelli, Italian hepatologist, studies on bilirubin
See also
- Babesiosis
- Biliary atresia
- Bilirubin diglucuronide
- Biliverdin
- Crigler–Najjar syndrome
- Gilbert's syndrome, a genetic disorder of bilirubin metabolism that can result in mild jaundice, found in about 5% of the population.
- Hy's Law
- Lumirubin
- Primary biliary cholangitis
- Primary sclerosing cholangitis
Notes
- ^ For conversion, 1 mg/dl = 17.1 μmol/L.
References
- S2CID 4278361.
- .
- ^ Braunstein E (3 May 2019). "Overview of Hemolytic Anemia – Hematology and Oncology". Merck Manuals Professional Edition (in Latin). Retrieved 5 May 2019.
- ^ "Bilirubin blood test", U.S. National Library of Medicine.
- ISBN 1-4160-2328-3
- S2CID 12394659.
- ISBN 978-0-7020-3367-4.
- ^ PMID 38172624.
- ISBN 978-0-7216-8178-8, retrieved 1 November 2023
- PMID 19206232.
- ^ ISBN 978-1-4160-3256-4, retrieved 31 October 2023
- PMID 7361112.
- ^ "Bilirubin's Chemical Formula". Archived from the original on 4 May 2011. Retrieved 14 August 2007.
- PMID 3029864.
- PMID 12456881.
- PMID 19286972.
- S2CID 25089098.
- PMID 31353321.
- ^ ISBN 978-0-323-47794-9, retrieved 31 October 2023
- ^ PMID 14765767.
- ^ "Sulfonamides: Bacteria and Antibacterial Drugs: Merck Manual Professional".[permanent dead link]
- PMID 29494090. Retrieved 3 May 2019.
- PMID 13508735.
- S2CID 30298986.
- ^ Merck Manual Jaundice Last full review/revision July 2009 by Steven K. Herrine
- PMID 32366186.
- S2CID 234775572. Retrieved 11 November 2021 – via PubMed.
- PMID 15173506.
- ^ "Neonatal Jaundice". Slhd.nsw.gov.au. 24 August 2009. Retrieved 16 March 2022.
- S2CID 43486067.
- PMID 18343383.
- Elsevier Science Direct.
- S2CID 230281925. Retrieved 11 November 2021 – via Elsevier Science Direct.
- PMC 2131702. Retrieved 24 March 2024.
- ^ "Bilirubin test: What you can expect". Mayo Clinic. 8 October 2022. Retrieved 24 March 2024.
- ^ "Newborn jaundice: Bilirubin test". National Health Service UK. 15 September 2017. Retrieved 24 March 2024.
- PMC 8114612. Retrieved 24 March 2024.
- labtestsonline.org. Retrieved 14 June 2017.
- ^ ISBN 978-0-323-07738-5.
- S2CID 78381597.
- ^ a b "Unconjugated Hyperbilirubinemia: Practice Essentials, Background, Pathophysiology". Medscape Reference. 4 March 2019. Retrieved 6 May 2019.
- ^ "Bilirubin: Reference Range, Interpretation, Collection and Panels". Medscape Reference. 1 February 2019. Retrieved 6 May 2019.
- ISBN 978-0199830121.
- ISBN 978-3-540-76838-8.
- ISBN 978-1-4377-0774-8.
- ISBN 978-1-4377-0755-7.
- ^ PMID 29261920. Retrieved 22 December 2019.
This fraction of conjugated bilirubin gets covalently bound to albumin, and is called delta bilirubin or delta fraction or biliprotein. As the delta bilirubin is bound to albumin, its clearance from serum takes about 12–14 days (equivalent to the half-life of albumin) in contrast to the usual 2 to 4 hours (half-life of bilirubin).
- PMID 13783422.
- PMID 11568098.
- ^ "SI Units". NIST. 12 April 2010.
- ^ MedlinePlus Encyclopedia: 003479
- ^ "Harmonisation of Reference Intervals" (PDF). Pathology Harmony. Archived from the original (PDF) on 18 December 2014. Retrieved 23 September 2014.
- ^ a b Golonka D. "Digestive Disorders Health Center: Bilirubin". WebMD. p. 3. Archived from the original on 1 January 2010. Retrieved 14 January 2010.
- ^ MedlinePlus Encyclopedia: CHEM-20
- ^ "Laboratory tests". Archived from the original on 13 August 2007. Retrieved 14 August 2007.
- S2CID 524952.
- ^ "Bilirubin - urine: MedlinePlus Medical Encyclopedia". medlineplus.gov. Retrieved 31 October 2023.
- ^ "Urinalysis: three types of examinations". Lab Tests Online (USA). Retrieved 16 August 2013.
- ^ PMID 21250145.
- ^ a b c d e f g h i j k Watson, Cecil J. (1977). "Historical Review of Bilirubin Chemistry". In Berk, Paul D. (ed.). International Symposium on Chemistry and Physiology of Bile Pigments. U.S. Department of Health, Education, and Welfare, Public Health Service, National Institutes of Health. pp. 3–16.
- ^ Hian Siong Leon Maria Tjen (30 January 1979). "Cholescintigraphy: The clinical application of 99mTechnetium-diethyl-IDA to the investigation of the liver and biliary tract. PhD thesis, Utrecht University" (PDF). Archived (PDF) from the original on 3 November 2021.
- ISBN 978-3-7091-1636-4.
- ^ Merriam-Webster, Merriam-Webster's Unabridged Dictionary, Merriam-Webster, archived from the original on 25 May 2020, retrieved 14 January 2018.
- ^ Elsevier, Dorland's Illustrated Medical Dictionary, Elsevier, archived from the original on 11 January 2014, retrieved 14 January 2018.
- ^ S2CID 233205099.
- ^ a b Berk, Paul D.; Berlin, Nathaniel I. (1977). International Symposium on Chemistry and Physiology of Bile Pigments. U.S. Department of Health, Education, and Welfare, Public Health Service, National Institutes of Health. pp. 27, 50.
- S2CID 4073510.
- ^ "Bilirubin". American Chemical Society. Retrieved 28 May 2021.
External links
- Bilirubin: analyte monograph from The Association for Clinical Biochemistry and Laboratory Medicine

