IDPN (chemical)
| |

| |
| Names | |
|---|---|
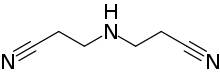
| Preferred IUPAC name
3,3′-Azanediyldipropanenitrile | |
| Other names
Bis(2-cyanoethyl)amine
| |
| Identifiers | |
3D model (
JSmol ) |
|
| ChemSpider | |
ECHA InfoCard
|
100.003.566 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| UN number | 3334 |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C6H9N3 | |
| Molar mass | 123.159 g·mol−1 |
| Density | 1.02 |
| Melting point | −5.5 °C (22.1 °F; 267.6 K) |
| Hazards | |
| GHS labelling: | |

| |
| Warning | |
| H315, H319, H335 | |
| P261, P264, P271, P280, P302+P352, P304+P340, P305+P351+P338, P312, P321, P332+P313, P337+P313, P362, P403+P233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
IDPN (3,3'-iminodipropanenitrile) is a neurotoxin with ototoxic and hepatotoxic effects. It causes irreversible movement disorder.[1][2][3]
Ototoxicity
IDPN has been shown to kill vestibular
vestibular function, in rats,[4] mice, guinea pigs, and frogs.[5] In rodents, the loss of vestibular function results in balance-related deficits, including circling behavior, retropulsion, and head bobbing, as well as weight loss.[5] Type I hair cells are more sensitive to IDPN toxicity than Type II hair cells.[4] No regeneration of vestibular hair cells was observed, thus these effects can be considered to be irreversible.[4]
IDPN has also been shown to kill cochlear hair cells, affecting auditory function.[6] IDPN-induced hearing loss covered a broad range of frequencies.
References
