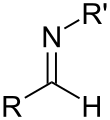
Imine

In organic chemistry, an imine (/ɪˈmiːn/ or /ˈɪmɪn/) is a functional group or organic compound containing a carbon–nitrogen double bond (C=N). The nitrogen atom can be attached to a hydrogen or an organic group (R). The carbon atom has two additional single bonds.[1][2] Imines are common in synthetic and naturally occurring compounds and they participate in many reactions.[3]
Structure
For ketimines and aldimines, respectively, the five core atoms (C2C=NX and C(H)C=NX, X = H or C) are coplanar. Planarity results from the sp2-hybridization of the mutually
Nomenclature and classification
The term "imine" was coined in 1883 by the German chemist Albert Ladenburg.[6]
Usually imines refer to compounds with the general formula R2C=NR, as discussed below.[7] In the older literature, imine refers to the aza-analogue of an epoxide. Thus, ethylenimine is the three-membered ring species aziridine C2H4NH.[8] The relationship of imines to amines having double and single bonds can be correlated with imides and amides, as in succinimide vs acetamide.
Imines are related to
A primary imine in which C is attached to both a hydrocarbyl and a H is called a primary aldimine; a secondary imine with such groups is called a secondary aldimine.[10] A primary imine in which C is attached to two hydrocarbyls is called a primary ketimine; a secondary imine with such groups is called a secondary ketimine.[11]
-
Primary aldimine,E-isomer
-
Secondary aldimine, E-isomer
-
Primary ketimine
-
Secondary ketimine
-
Aziridine and its derivatives are sometimes referred to as imines.
Synthesis of imines
Carbonyl-amine condensation
Imines are typically prepared by the condensation of primary amines and aldehydes.
Rarer than primary amines is the use of ammonia to give a primary imine.[17] In the case of hexafluoroacetone, the hemiaminal intermediate can be isolated.[18]
From nitriles
- C6H5CN + C6H5MgBr → (C6H5)2C=NMgBr
- (C6H5)2C=NMgBr + H2O → (C6H5)2C=NH + MgBr(OH)
Specialized methods
Several other methods exist for the synthesis of imines.
- Reaction of organic azides with metal carbenoids (produced from diazocarbonyl compounds).[23]
- Condensation of carbon acids with nitrosocompounds.
- The rearrangement of trityl N-haloamines in the Stieglitz rearrangement.
- By reaction of alkenes with hydrazoic acid in the Schmidt reaction.
- By reaction of a nitrile, hydrochloric acid, and an arene in the Hoesch reaction.
- Multicomponent synthesis of 3-thiazolines in the Asinger reaction.
- Thermal decomposition of oximes.[24]
Reactions

Hydrolysis
The chief reaction of imines, often undesirable, is their hydrolysis back to the amine and the carbonyl precursor.
- R2C=NR' + H2O ⇌ R2C=O + R'NH2
Precursors to heterocycles
Imines are widely used as intermediates in the synthesis of heterocycles.
- Aromatic imines react with an enol ether to a quinoline in the Povarov reaction.
- Imines react, thermally, with
- Imine react with dienes in the Imine Diels-Alder reaction to a tetrahydropyridine.
- allylic amines in the Aza-Baylis–Hillman reaction.
Acid-base reactions
Somewhat like the parent amines, imines are mildly basic and reversibly protonate to give iminium salts:
- R2C=NR' + H+ [R2C=NHR']+
Alternatively, primary imines are sufficiently acidic to allow N-alkylation, as illustrated with benzophenone imine:[28]
- (C6H5)2C=NH + CH3Li → (C6H5)2C=NLi + CH4
- (C6H5)2C=NLi + CH3I → (C6H5)2C=NCH3 + LiI
Lewis acid-base reactions
Imines are common
Nucleophilic additions
Very analogous to ketones and aldehydes, primary imines are susceptible to attack by carbanion equivalents. The method allow for the synthesis of secondary amines:[29][30]
- R2C=NR' + R"Li → R2R"CN(Li)R'
- R2R"CN(Li)R' + H2O → R2R"CNHR' + LiOH
Imine reductions
Imines are reduced via reductive amination. An imine can be reduced to an amine via hydrogenation for example in a synthesis of m-tolylbenzylamine:[31]
Other reducing agents are lithium aluminium hydride and sodium borohydride.[32]
The
Owing to their enhanced electrophilicity, iminium derivatives are particularly susceptible to reduction to the amines. Such reductions can be achieved by
Polymerisation
Unhindered aldimines tend to cyclize, as illustrated by the condensation of methylamine and formaldehyde, which gives the hexahydro-1,3,5-triazine.
Imine polymers (
Miscellaneous reactions
Akin to
Imine are oxidized with
Imines are intermediates in the alkylation of amines with formic acid in the
A rearrangement in carbohydrate chemistry involving an imine is the Amadori rearrangement.
A methylene transfer reaction of an imine by an unstabilised sulphonium ylide can give an aziridine system. Imine react with dialkylphosphite in the Pudovik reaction and Kabachnik–Fields reaction
Biological role
Imines are common in nature.[40][41] The pyridoxal phosphate-dependent enzymes (PLP enzymes) catalyze myriad reactions involving aldimines (or Schiff bases).[42] Cyclic imines are also substrates for many imine reductase enzymes.[43]

See also
- Enamine
- Schiff base
- Carboximidate
- Oxazolidine
- Other functional groups with a C=N double bond: oximes, hydrazones
- Other functional groups with a C N triple bond: isonitriles
References
- OCLC 642506595.
- OCLC 639112179.
- ISBN 9780470771204.
- .
- .
From p. 1150: Denn offenbar gehört auch das Piperidin in die Klasse der von mir gesuchten Verbindungen, für welche der Name Imine durch die bestehende Nomenklatur angezeigt ist.
[For obviously piperidine also belongs in the class of compounds that are sought by me, for which the name "imines" is indicated by the prevailing nomenclature.] - OCLC 922539.
- .
- .
- .
- PMID 25906082.
- PMID 24359453.
- PMID 22694241.
- .
- ^ .
- ISBN 9780470638859.
- .
- ^ Moureu, Charles; Mignonac, Georges (1920). "Les Cetimines". Annales de Chimie. 9 (13): 322–359. Retrieved 18 June 2014.
- ISSN 0022-3263.
- PMID 24423056.
- .
- .
- .
- .
- .
- S2CID 235020219.
- doi:10.15227/orgsyn.084.0306.)
{{cite journal}}: CS1 maint: multiple names: authors list (link - ^ C. F. H. Allen and James VanAllan (1955). "m-Tolylbenzylamine". Organic Syntheses: 827; Collected Volumes, vol. 3.
- ^ For example: Ieva R. Politzer and A. I. Meyers (1988). "Aldehydes from 2-Benzyl-4,4,6-trimethyl-5,6-dihydro-1,3(4H)-oxazine: 1-Phenylcyclopentanecarboxaldehyde". Organic Syntheses; Collected Volumes, vol. 6, p. 905.
- .
- PMID 11749440.
- ISBN 3-13-106124-3.
- .
- PMID 34163597.
- S2CID 214199868.
- .
- ^ "Researchers look to nature to unearth the secrets of cyclic imine cleavage". EurekAlert!. Retrieved 2021-07-22.
- PMID 31103411.
- PMID 15189147.
- PMID 28038349.