Interferon beta-1a
 | |
| Clinical data | |
|---|---|
| Trade names | Avonex, Rebif, Plegridy, others |
| AHFS/Drugs.com | Professional Drug Facts |
| MedlinePlus | a604005 |
| License data |
|
| Pregnancy category |
|
Subcutaneous, intramuscular | |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Elimination half-life | 10 hrs |
| Identifiers | |
| |
| CAS Number | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider |
|
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C908H1408N246O252S7 |
| Molar mass | 20027.14 g·mol−1 |
| | |
Interferon beta-1a (also interferon beta 1-alpha) is a
Interferon beta has not been shown to slow the advance of disability.[8][9][10][11] Interferons are not a cure for MS (there is no known cure); the claim is that interferons may slow the progress of the disease if started early and continued for the duration of the disease.[12]
Medical uses
Clinically isolated syndrome
The earliest clinical presentation of relapsing-remitting multiple sclerosis is the clinically isolated syndrome (CIS), that is, a single attack of a single symptom. During a CIS, there is a subacute attack suggestive of
Relapsing-remitting MS
Medications are modestly effective at decreasing the number of attacks in relapsing-remitting multiple sclerosis
While more studies of the long-term effects of the drugs are needed,[12][14] existing data on the effects of interferons indicate that early-initiated long-term therapy is safe and it is related to better outcomes.[12]
Side effects
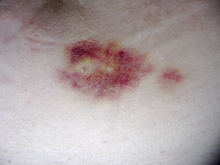
Interferon beta-1a is available only in injectable forms, and can cause skin reactions at the injection site that may include cutaneous necrosis. Skin reactions with interferon beta are more common with subcutaneous administration and vary greatly in their clinical presentation.[20] They usually appear within the first month of treatment albeit their frequence and importance diminish after six months of treatment.[20] Skin reactions are more prevalent in women.[20] Mild skin reactions usually do not impede treatment whereas necroses appear in around 5% of patients and lead to the discontinuation of the therapy.[20] Also over time, a visible dent at the injection site due to the local destruction of fat tissue, known as lipoatrophy, may develop, however, this rarely occurs with interferon treatment.[21]
To help prevent injection-site reactions, patients are advised to rotate injection sites and use an aseptic injection technique. Injection devices are available to optimize the injection process. Side effects are often onerous enough that many patients ultimately discontinue taking interferons [citation needed] (or glatiramer acetate, a comparable disease-modifying therapy requiring regular injections).
Mechanism of action
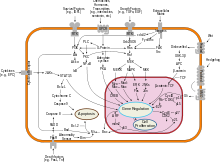
Interferon beta balances the expression of pro- and anti-inflammatory agents in the brain, and reduces the number of inflammatory cells that cross the
Society and culture
Brand names
Avonex
Avonex was approved in the US in 1996,
Avonex is sold in three formulations, a lyophilized powder requiring reconstitution, a pre-mixed liquid syringe kit, and a pen; it is administered via intramuscular injection.[1]
Rebif
Rebif is a disease-modifying drug (DMD) used to treat multiple sclerosis in cases of clinically isolated syndromes as well as relapsing forms of multiple sclerosis and is similar to the interferon beta protein produced by the human body. It is co-marketed by
Cinnovex
Cinnovex is the brand name of recombinant Interferon beta-1a, which is manufactured as biosimilar/biogeneric in
Plegridy
Plegridy is a brand name of a
Betaferon (interferon beta-1b)
Closely related to interferon beta-1a is interferon beta-1b, which is also indicated for MS, but is formulated with a different dose and administered with a different frequency. Each drug has a different safety/efficacy profile.[30] Interferon beta-1b is marketed only by Bayer in the US as Betaseron, and outside the US as Betaferon.
Economics
In the United States, as of 2015[update], the cost is between US$1,284 and US$1,386 per 30 mcg vial.[31] As of 2020, the National Average Drug Acquisition Cost (NADAC) in the United States for Avonex was $6,872.94 for a 30 mcg kit.[32]
Avonex and Rebif are on the top ten best-selling multiple sclerosis drugs of 2013.[33]
It is an example of a
| No. | 2013 Global Sales | INN |
Brand names | Companies |
|---|---|---|---|---|
| 1 | $4.33 billion | Glatiramer acetate | Copaxone | Teva |
| 2 | $3.00 billion | Interferon beta 1a | Avonex | Biogen Idec |
| 3 | $2.51 billion | Interferon beta 1a | Rebif | Merck KGaA |
| 4 | $1.93 billion | Fingolimod | Gilenya | Novartis |
| 5 | $1.41 billion | Natalizumab | Tysabri | Biogen Idec |
| 6 | $1.38 billion | Interferon beta 1b |
Betaseron/Betaferon | Bayer HealthCare |
| 7 | $876 million | Dimethyl fumarate | Tecfidera | Biogen Idec |
| 8 | $303 million | 4-Aminopyridine | Ampyra | Acorda Therapeutics |
| 9 | $250 million | Adrenocorticotropic hormone | H.P. Acthar Gel | Questcor Pharmaceuticals |
| 10 | $221 million | Teriflunomide | Aubagio | Sanofi |
Research
COVID-19
Interferon beta-1a administered subcutaneously or intravenously was investigated since March 2020 as a potential treatment in patients hospitalized with COVID-19 in a multinational Solidarity trial (initially in combination with lopinavir) but it did not reduce in-hospital mortality compared to local standard of care.[35]
SNG001, an inhalation formulation of interferon beta-1a, is being developed as a treatment for COVID-19 by Synairgen.[36][37] A pilot trial in hospitalized patients showed higher odds of clinical improvement with SNG001 compared to placebo[38] and in January 2021 a phase 3 trial in this population started.[39]
References
- ^ a b "Avonex- interferon beta-1a kit Avonex Pen- interferon beta-1a injection, solution Avonex- interferon beta-1a injection, solution". DailyMed. 30 March 2020. Retrieved 1 October 2020.
- ^ a b "Rebif- interferon beta-1a kit Rebif Rebidose- interferon beta-1a kit Rebif- interferon beta-1a injection, solution Rebif- interferon beta-1a injection, solution Rebif Rebidose- interferon beta-1a injection, solution". DailyMed. 5 June 2020. Retrieved 1 October 2020.
- ^ "Avonex EPAR". European Medicines Agency (EMA). 17 September 2018. Retrieved 1 October 2020.
- ^ "Rebif EPAR". European Medicines Agency (EMA). 17 September 2018. Retrieved 1 October 2020.
- S2CID 3122427.
- PMID 12397132.
- ^ Stachowiak J (2008). "Is Avonex Right for You?". Archived from the original on 2016-03-03. Retrieved 2008-05-07.
- PMID 22797642.
- PMID 26374702.
- S2CID 45183415.
- S2CID 24663294.
- ^ S2CID 16929304.
- PMID 24871874.
- ^ S2CID 195686659.
- S2CID 362182.
- PMID 11687131.
- ^ S2CID 19618597.
- S2CID 36392563.
- S2CID 40367120.
- ^ S2CID 30330292.
- PMID 15038472.
- PMID 16253889.
- ^ S2CID 25516515.
- S2CID 42050086.
- ^ "F.D.A. Approves a Biogen Drug for Treating Multiple Sclerosis". The New York Times. 8 May 1996. Retrieved 16 January 2021.
- ^ "Neurology & Immunology". EMD Group.
- ^ "EMD Serono Takes on Exclusive Promotion of Rebif (interferon beta-1a) in the US" (Press release). EMD Serono – via PR Newswire.
- S2CID 9236986.
- ^ "Plegridy- peginterferon beta-1a kit Plegridy Pen- peginterferon beta-1a kit Plegridy- peginterferon beta-1a injection, solution". DailyMed. 19 July 2019. Retrieved 30 March 2020.
- PMID 21095482.
- ^ Langreth R (June 29, 2016). "Decoding Big Pharma's Secret Drug Pricing Practices". Bloomberg. Retrieved 15 July 2016.
- ^ "NADAC as of 2020-02-12". Centers for Medicare and Medicaid Services. Archived from the original on 2020-02-18. Retrieved 2020-02-18.
- ^ "The top 10 best-selling multiple sclerosis drugs of 2013". FiercePharma. 9 September 2014.
- PMID 23964615.
- PMID 33264556.
- ^ "COVID Trial at Home - developed by Synairgen". Covid Trial at Home - developed by Synairgen.
- ^ "University-led COVID19 drug trial expands into home testing". medicalxpress.
- PMID 33189161.
- ^ "Synairgen begins large-scale trial of inhaled COVID-19 treatment". PharmaTimes. 13 January 2021. Retrieved 7 August 2021.
External links
- "Interferon beta-1a". Drug Information Portal. U.S. National Library of Medicine.
- "Interferon Beta-1a Intramuscular Injection". MedlinePlus.
