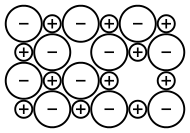
Salt (chemistry)
In
The component ions in a salt can be either
4), and ammonium (NH+
4) and carbonate (CO2−
3) ions in ammonium carbonate. Salt containing basic ions hydroxide (OH−) or oxide (O2−) are classified as bases, for example sodium hydroxide
Individual ions within a salt usually have multiple near neighbours, so are not considered to be part of molecules, but instead part of a continuous three-dimensional network. Salts usually form crystalline structures when solid.
Salts composed of small ions typically have high
History of discovery

In 1913 the structure of sodium chloride was determined by
Principal contributors to the development of a theoretical treatment of ionic crystal structures were Max Born, Fritz Haber, Alfred Landé, Erwin Madelung, Paul Peter Ewald, and Kazimierz Fajans.[6] Born predicted crystal energies based on the assumption of ionic constituents, which showed good correspondence to thermochemical measurements, further supporting the assumption.[4]
Formation


Many metals such as the alkali metals react directly with the electronegative halogens gases to salts.[7][8]
Salts form upon evaporation of their solutions.[9] Once the solution is supersaturated and the solid compound nucleates.[9] This process occurs widely in nature and is the means of formation of the evaporite minerals.[10]
Insoluble ionic compounds can be precipitated by mixing two solutions, one with the cation and one with the anion in it. Because all solutions are electrically neutral, the two solutions mixed must also contain
If the solvent is water in either the evaporation or precipitation method of formation, in many cases the ionic crystal formed also includes water of crystallization, so the product is known as a hydrate, and can have very different chemical properties compared to the anhydrous material.[13]
Molten salts will solidify on cooling to below their
In some reactions between highly reactive metals (usually from Group 1 or Group 2) and highly electronegative halogen gases, or water, the atoms can be ionized by electron transfer,[16] a process thermodynamically understood using the Born–Haber cycle.[17]
Salts are formed by salt-forming reactions
- A base and an acid, e.g., NH3 + HCl → NH4Cl
- A metal and an acid, e.g., Mg + H2SO4 → MgSO4 + H2
- A metal and a non-metal, e.g., Ca + Cl2 → CaCl2
- A
- An acid and a base anhydride, e.g., 2 HNO3 + Na2O → 2 NaNO3 + H2O
- In the salt metathesis reaction where two different salts are mixed in water, their ions recombine, and the new salt is insoluble and precipitates. For example:
- Pb(NO3)2 + Na2SO4 → PbSO4↓ + 2 NaNO3
Bonding
Ions in ionic compounds are primarily held together by the
If the
Although chemists classify idealized bond types as being ionic or covalent, the existence of additional types such as
Structure
The lattice energy is the summation of the interaction of all sites with all other sites. For unpolarizable spherical ions, only the charges and distances are required to determine the electrostatic interaction energy. For any particular ideal crystal structure, all distances are geometrically related to the smallest internuclear distance. So for each possible crystal structure, the total electrostatic energy can be related to the electrostatic energy of unit charges at the nearest neighboring distance by a multiplicative constant called the
Using an even simpler approximation of the ions as impenetrable hard spheres, the arrangement of anions in these systems are often related to close-packed arrangements of spheres, with the cations occupying tetrahedral or octahedral interstices.[34][35] Depending on the stoichiometry of the ionic compound, and the coordination (principally determined by the radius ratio) of cations and anions, a variety of structures are commonly observed,[36] and theoretically rationalized by Pauling's rules.[37]
| Stoichiometry | Cation:anion coordination |
Interstitial sites | Cubic close packing of anions | Hexagonal close packing of anions | |||
|---|---|---|---|---|---|---|---|
| Occupancy | Critical radius ratio |
Name | Madelung constant | Name | Madelung constant | ||
| MX | 6:6 | all octahedral | 0.4142[34] | sodium chloride | 1.747565[38] | nickeline | <1.73[a][39] |
| 4:4 | alternate tetrahedral | 0.2247[40] | zinc blende |
1.6381[38] | wurtzite | 1.641[4] | |
| MX2 | 8:4 | all tetrahedral | 0.2247 | fluorite | 5.03878[41] | ||
| 6:3 | half octahedral (alternate layers fully occupied) | 0.4142 | cadmium chloride | 5.61[42] | cadmium iodide | 4.71[41] | |
| MX3 | 6:2 | one-third octahedral | 0.4142 | rhodium(III) bromide[b][43][44] | 6.67[45][c] | bismuth iodide |
8.26[45][d] |
| M2X3 | 6:4 | two-thirds octahedral | 0.4142 | corundum | 25.0312[41] | ||
| ABO3 | two-thirds octahedral | 0.4142 | ilmenite | Depends on charges and structure [e] | |||
| AB2O4 | one-eighth tetrahedral and one-half octahedral | rA/rO = 0.2247, rB/rO = 0.4142[f] |
spinel, inverse spinel | Depends on cation site distributions[48][49][50] |
olivine | Depends on cation site distributions[51] | |
In some cases, the anions take on a simple cubic packing and the resulting common structures observed are:
| Stoichiometry | Cation:anion coordination |
Interstitial sites occupied | Example structure | ||
|---|---|---|---|---|---|
| Name | Critical radius ratio |
Madelung constant | |||
| MX | 8:8 | entirely filled | cesium chloride |
0.7321[52] | 1.762675[38] |
| MX2 | 8:4 | half filled | calcium fluoride | ||
| M2X | 4:8 | half filled | lithium oxide | ||
Some ionic liquids, particularly with mixtures of anions or cations, can be cooled rapidly enough that there is not enough time for crystal nucleation to occur, so an ionic glass is formed (with no long-range order).[53]
Defects
Within any crystal, there will usually be some defects. To maintain electroneutrality of the crystals, defects that involve loss of a cation will be associated with loss of an anion, i.e. these defects come in pairs.
Properties
Acidity/basicity
Ionic compounds containing
Some ions are classed as amphoteric, being able to react with either an acid or a base.[59] This is also true of some compounds with ionic character, typically oxides or hydroxides of less-electropositive metals (so the compound also has significant covalent character), such as zinc oxide, aluminium hydroxide, aluminium oxide and lead(II) oxide.[60]
Melting and boiling points
Electrostatic forces between particles are strongest when the charges are high, and the distance between the nuclei of the ions is small. In such cases, the compounds generally have very high melting and boiling points and a low vapour pressure.[61] Trends in melting points can be even better explained when the structure and ionic size ratio is taken into account.[62] Above their melting point, ionic solids melt and become molten salts (although some ionic compounds such as aluminium chloride and iron(III) chloride show molecule-like structures in the liquid phase).[63] Inorganic compounds with simple ions typically have small ions, and thus have high melting points, so are solids at room temperature. Some substances with larger ions, however, have a melting point below or near room temperature (often defined as up to 100 °C), and are termed ionic liquids.[64] Ions in ionic liquids often have uneven charge distributions, or bulky substituents like hydrocarbon chains, which also play a role in determining the strength of the interactions and propensity to melt.[65]
Even when the local structure and bonding of an ionic solid is disrupted sufficiently to melt it, there are still strong long-range electrostatic forces of attraction holding the liquid together and preventing ions boiling to form a gas phase.[66] This means that even room temperature ionic liquids have low vapour pressures, and require substantially higher temperatures to boil.[66] Boiling points exhibit similar trends to melting points in terms of the size of ions and strength of other interactions.[66] When vapourized, the ions are still not freed of one another. For example, in the vapour phase sodium chloride exists as diatomic "molecules".[67]
Brittleness
Most ionic compounds are very
Compressibility
The compressibility of an ionic compound is strongly determined by its structure, and in particular the coordination number. For example, halides with the caesium chloride structure (coordination number 8) are less compressible than those with the sodium chloride structure (coordination number 6), and less again than those with a coordination number of 4.[70]
Solubility

When simple salts
The
The
Electrical conductivity
Salts are characteristically
In some unusual ionic compounds:
Colour
The
The anions in compounds with bonds with the most ionic character tend to be colorless (with an absorption band in the ultraviolet part of the spectrum).[81] In compounds with less ionic character, their color deepens through yellow, orange, red, and black (as the absorption band shifts to longer wavelengths into the visible spectrum). [81]
The absorption band of simple cations shifts toward a shorter wavelength when they are involved in more covalent interactions.[81] This occurs during hydration of metal ions, so colorless anhydrous ionic compounds with an anion absorbing in the infrared can become colorful in solution.[81]
Salts exist in many different colors, which arise either from their constituent anions, cations or solvates. For example:
- chromate ionCrO2−4.
- dichromate ionCr2O2−7.
- cobalt(II) nitrate hexahydrate Co(NO3)2·6H2O is made red by the chromophore of hydrated cobalt(II) [Co(H2O)6]2+.
- copper(II) sulfate pentahydrate CuSO4·5H2O is made blue by the hydrated copper(II) cation.
- potassium permanganate KMnO4 is made violet by the permanganate anion MnO−4.
- nickel(II) chloride hexahydrate NiCl2·6H2O is made green by the hydrated nickel(II) chloride [NiCl2(H2O)4].
- anionsdo not absorb light in the part of the spectrum that is visible to humans.
Some
Taste and odor
Salts can elicit all five
).Salts of strong acids and strong bases ("
Uses
Ionic compounds have long had a wide variety of uses and applications. Many
Soluble ionic compounds like salt can easily be dissolved to provide
The chemical identity of the ions added is also important in many uses. For example, fluoride containing compounds are dissolved to supply fluoride ions for water fluoridation.[88]
Solid ionic compounds have long been used as paint pigments, and are resistant to organic solvents, but are sensitive to acidity or basicity.[89] Since 1801 pyrotechnicians have described and widely used metal-containing ionic compounds as sources of colour in fireworks.[90] Under intense heat, the electrons in the metal ions or small molecules can be excited.[91] These electrons later return to lower energy states, and release light with a colour spectrum characteristic of the species present.[92][93]
In chemistry, ionic compounds are often used as precursors for high-temperature solid-state synthesis.[94]
Many metals are geologically most abundant as ionic compounds within
Nomenclature
According to the
4, sulfate, is an example of a polyatomic ion). To obtain the empirical formula from these names, the stoichiometry can be deduced from the charges on the ions, and the requirement of overall charge neutrality.[102]
If there are multiple different cations and/or anions, multiplicative prefixes (di-, tri-, tetra-, ...) are often required to indicate the relative compositions,
Compounds containing one or more elements which can exist in a variety of charge/
Common salt-forming cations include:
- Ammonium NH+
4 - Calcium Ca2+
- Iron Fe2+
and Fe3+ - Magnesium Mg2+
- Potassium K+
- Pyridinium C
5H
5NH+ - arylgroup
- Sodium Na+
- Copper Cu2+
Common salt-forming anions (parent acids in parentheses where available) include:
- Acetate CH
3COO−
(acetic acid) - Carbonate CO2−
3 (carbonic acid) - Chloride Cl−
(hydrochloric acid) - Citrate HOC(COO−)
)(CH
2COO−
)
2 (citric acid - hydrocyanic acid)
- Fluoride F−
(hydrofluoric acid) - Nitrate NO−
3 (nitric acid) - Nitrite NO−
2 (nitrous acid) - Oxide O2−
(water) - Phosphate PO3−
4 (phosphoric acid) - Sulfate SO2−
4 (sulfuric acid)
Salts with varying number of hydrogen atoms replaced by cations as compared to their parent acid can be referred to as monobasic, dibasic, or tribasic, identifying that one, two, or three hydrogen atoms have been replaced; polybasic salts refer to those with more than one hydrogen atom replaced. Examples include:
- Sodium phosphate monobasic (NaH2PO4)
- Sodium phosphate dibasic (Na2HPO4)
- Sodium phosphate tribasic (Na3PO4)
Types of salt
Acidity and basicity
Salts can be classified in a variety of ways. Salts that produce
Strength
Strong salts or strong electrolyte salts are chemical salts composed of strong electrolytes. These salts dissociate completely or almost completely in water. They are generally odorless and nonvolatile.
Strong salts start with Na__, K__, NH4__, or they end with __NO3, __ClO4, or __CH3COO. Most group 1 and 2 metals form strong salts. Strong salts are especially useful when creating conductive compounds as their constituent ions allow for greater conductivity.[citation needed]
Weak salts or weak electrolyte salts are composed of weak electrolytes. These salts do not dissociate well in water. They are generally more volatile than strong salts. They may be similar in odor to the acid or base they are derived from. For example, sodium acetate, CH3COONa, smells similar to acetic acid CH3COOH.
See also
- Bonding in solids
- Ioliomics
- Salt metathesis reaction
- Bresle method (the method used to test for salt presence during coating applications)
- Carboxylate
- Halide
- Ionic bonds
- Natron
- Salinity
Notes
- ^ This structure type has a variable lattice parameter c/a ratio, and the exact Madelung constant depends on this.
- ^ This structure has been referred to in references as yttrium(III) chloride and chromium(III) chloride, but both are now known as the RhBr3 structure type.
- ^ The reference lists this structure as MoCl3, which is now known as the RhBr3 structure.
- ^ The reference lists this structure as FeCl3, which is now known as the BiI3 structure type.
- ^ This structure type can accommodate any charges on A and B that add up to six. When both are three the charge structure is equivalent to that of corrundum.[46] The structure also has a variable lattice parameter c/a ratio, and the exact Madelung constant depends on this.
- ^ However, in some cases such as MgAl2O4 the larger cation occupies the smaller tetrahedral site.[47]
References
- S2CID 13112732.
- .
- ^ .
- .
- ^ Pauling 1960, p. 505.
- ^ Zumdahl 1989, p. 312.
- ^ a b c Wold & Dwight 1993, p. 71.
- ^ a b Wold & Dwight 1993, p. 82.
- ISBN 978-0-521-52958-7. Archivedfrom the original on 2017-12-03.
- ^ a b Zumdahl 1989, p. 133–140.
- ^ Zumdahl 1989, p. 144–145.
- ^ a b Brown 2009, p. 417.
- ^ Wold & Dwight 1993, p. 79.
- ^ Wold & Dwight 1993, pp. 79–81.
- ^ Zumdahl 1989, p. 312–313.
- ^ Barrow 1988, p. 161–162.
- ^ Pauling 1960, p. 6.
- ^ Kittel 2005, p. 61.
- ^ a b c Pauling 1960, p. 507.
- ^ Ashcroft & Mermin 1977, p. 379.
- ^ a b Pauling 1960, p. 65.
- .
- PMID 18893624. Archived from the originalon 2021-12-07. Retrieved 2021-12-01.
- ISBN 978-0-470-56753-1.
- .
- .
- ^ Barrow 1988, p. 676.
- S2CID 120135228.
- ^ Seifert, Vanessa (27 November 2023). "Do bond classifications help or hinder chemistry?". chemistryworld.com. Retrieved 22 January 2024.
- ^ Kittel 2005, p. 64.
- ^ Pauling 1960, p. 509.
- ^ Carter, Robert (2016). "Lattice Energy" (PDF). CH370 Lecture Material. Archived (PDF) from the original on 2015-05-13. Retrieved 2016-01-19.
- ^ a b Ashcroft & Mermin 1977, p. 383.
- ^ Zumdahl 1989, p. 444–445.
- ^ ISBN 978-0-7487-7516-3.)
{{cite book}}: CS1 maint: multiple names: authors list (link - ^ Ashcroft & Mermin 1977, pp. 382–387.
- ^ a b c Kittel 2005, p. 65.
- .
- ^ Ashcroft & Mermin 1977, p. 386.
- ^ ISBN 978-0-12-118420-9.)
{{cite book}}: CS1 maint: multiple names: authors list (link - .
- ^ "YCl3 – Yttrium trichloride". ChemTube3D. University of Liverpool. 2008. Archived from the original on 27 January 2016. Retrieved 19 January 2016.
- ^ ISBN 978-0-8412-2725-5.
- ^ .
- ISBN 978-81-87224-70-9.
- ^ Wenk & Bulakh 2004, p. 778.
- .
- .
- .
- S2CID 101158673.
- ^ Ashcroft & Mermin 1977, p. 384.
- ^ .
- ^ .
- ^ ISBN 978-81-219-0263-2.
- ^ Kittel 2005, p. 376.
- ^ "Periodic Trends and Oxides". Archived from the original on 2015-12-29. Retrieved 2015-11-10.
- ISBN 978-0-03-072373-5.
- .
- ISBN 978-0-19-964182-6.
- ^ McQuarrie & Rock 1991, p. 503.
- ISSN 0002-7863.
- ISBN 978-94-010-0458-9. Archivedfrom the original on 2017-12-03.
- ^ Freemantle 2009, p. 1.
- ^ Freemantle 2009, pp. 3–4.
- ^ PMID 16851662.
- ISBN 978-0-323-13894-9. Archivedfrom the original on 2017-12-03.
- ^ .
- ISSN 0031-8086.
- doi:10.1021/ed014p34.
- ^ Brown 2009, pp. 89–91.
- ^ Brown 2009, pp. 413–415.
- ^ a b Brown 2009, p. 422.
- .
- ^ "Electrical Conductivity of Ionic Compound". 2011-05-22. Archived from the original on 21 May 2014. Retrieved 2 December 2012.
- ^ Zumdahl 1989, p. 341.
- ^ ISBN 978-981-02-3473-7. Archivedfrom the original on 2017-12-03.
- .
- .
- ^ Pauling 1960, p. 105.
- ^ a b c d Pauling 1960, p. 107.
- ^ Wenk & Bulakh 2004, p. 774.
- ISBN 978-0-09-928199-3.
- ^ Lower, Simon (2014). "Naming Chemical Substances". Chem1 General Chemistry Virtual Textbook. Archived from the original on 16 January 2016. Retrieved 14 January 2016.
- ^ Atkins & de Paula 2006, pp. 150–157.
- ^ Atkins & de Paula 2006, pp. 761–770.
- ^ Atkins & de Paula 2006, pp. 163–169.
- ^ Reeves TG (1986). "Water fluoridation: a manual for engineers and technicians" (PDF). Centers for Disease Control. Archived from the original (PDF) on 2017-02-08. Retrieved 2016-01-18.
- ISBN 978-81-7141-276-1. Archivedfrom the original on 2017-12-03.
- ^ Russell 2009, p. 14.
- ^ Russell 2009, p. 82.
- ^ Russell 2009, pp. 108–117.
- ^ Russell 2009, pp. 129–133.
- ISBN 978-0-444-53599-3.
- ^ Zumdahl & Zumdahl 2015, pp. 822.
- ^ Zumdahl & Zumdahl 2015, pp. 823.
- ISBN 978-3-527-60525-5.
- ^ IUPAC 2005, p. 68.
- ^ IUPAC 2005, p. 70.
- ^ IUPAC 2005, p. 69.
- ISBN 978-0-534-99766-3.
- ^ Brown 2009, pp. 36–37.
- ^ IUPAC 2005, pp. 75–76.
- ^ IUPAC 2005, p. 75.
- doi:10.1139/v75-015.
- ^ IUPAC 2005, p. 76.
- ^ IUPAC 2005, pp. 76–77.
- ^ a b c d e IUPAC 2005, p. 77.
- ^ IUPAC 2005, pp. 77–78.
- .
- ^ a b Brown 2009, p. 38.
- ISBN 9780471193500. Archived from the originalon 2007-09-11.
- ISBN 0-14-200161-9.
Bibliography
- ISBN 978-0-03-083993-1.
- Atkins, Peter; de Paula, Julio (2006). Atkins' physical chemistry (8th ed.). Oxford: Oxford University Press. ISBN 978-0-19-870072-2.
- Barrow, Gordon M. (1988). Physical chemistry (5th ed.). New York: McGraw-Hill. ISBN 978-0-07-003905-6.
- Brown, Theodore L.; LeMay, H. Eugene Jr; Bursten, Bruce E.; Lanford, Steven; Sagatys, Dalius; Duffy, Neil (2009). Chemistry: the central science: a broad perspective (2nd ed.). Frenchs Forest, N.S.W.: Pearson Australia. ISBN 978-1-4425-1147-7.
- Freemantle, Michael (2009). An introduction to ionic liquids. Cambridge: Royal Society of Chemistry. ISBN 978-1-84755-161-0.
- International Union of Pure and Applied Chemistry, Division of Chemical Nomenclature (2005). Neil G. Connelly (ed.). Nomenclature of inorganic chemistry: IUPAC recommendations 2005 (New ed.). Cambridge: RSC Publ. ISBN 978-0-85404-438-2. Archived from the originalon 2016-02-03. Retrieved 2023-02-05.
- ISBN 978-0-471-41526-8.
- McQuarrie, Donald A.; Rock, Peter A. (1991). General chemistry (3rd ed.). New York: W.H. Freeman and Co. ISBN 978-0-7167-2169-7.
- ISBN 978-0-8014-0333-0.
- Russell, Michael S. (2009). The chemistry of fireworks (2nd ed.). Cambridge, UK: RSC Pub. ISBN 978-0-85404-127-5.
- Wenk, Hans-Rudolph; Bulakh, Andrei (2004). Minerals: Their Constitution and Origin (1st ed.). New York: Cambridge University Press. ISBN 978-1-107-39390-5.
- Wold, Aaron; Dwight, Kirby (1993). Solid State Chemistry Synthesis, Structure, and Properties of Selected Oxides and Sulfides. Dordrecht: Springer Netherlands. ISBN 978-94-011-1476-9.
- Zumdahl, Steven S. (1989). Chemistry (2nd ed.). Lexington, Mass.: D.C. Heath. ISBN 978-0-669-16708-5.
- Zumdahl, Steven; Zumdahl, Susan (2015). Chemistry: An Atoms First Approach. Cengage Learning. ISBN 978-1-305-68804-9.