Iron
 | ||||||||||||||||||||||||||||||||||||||||||||||
| Iron | ||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pronunciation | /ˈaɪərn/ | |||||||||||||||||||||||||||||||||||||||||||||
| Allotropes | see Allotropes of iron | |||||||||||||||||||||||||||||||||||||||||||||
| Appearance | lustrous metallic with a grayish tinge | |||||||||||||||||||||||||||||||||||||||||||||
| Standard atomic weight Ar°(Fe) | ||||||||||||||||||||||||||||||||||||||||||||||
| Iron in the periodic table | ||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||
kJ/mol | ||||||||||||||||||||||||||||||||||||||||||||||
| Heat of vaporization | 340 kJ/mol | |||||||||||||||||||||||||||||||||||||||||||||
| Molar heat capacity | 25.10 J/(mol·K) | |||||||||||||||||||||||||||||||||||||||||||||
Vapor pressure
| ||||||||||||||||||||||||||||||||||||||||||||||
| Atomic properties | ||||||||||||||||||||||||||||||||||||||||||||||
5000 BC | ||||||||||||||||||||||||||||||||||||||||||||||
| Symbol | "Fe": from Latin ferrum | |||||||||||||||||||||||||||||||||||||||||||||
| Isotopes of iron | ||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||
Iron is a
Extracting usable metal from
Pristine and smooth pure iron surfaces are a mirror-like silvery-gray. Iron reacts readily with oxygen and
have substantial industrial, medical, or research applications.The body of an adult human contains about 4 grams (0.005% body weight) of iron, mostly in
Characteristics
Allotropes

At least four allotropes of iron (differing atom arrangements in the solid) are known, conventionally denoted
.The first three forms are observed at ordinary pressures. As molten iron cools past its freezing point of 1538 °C, it crystallizes into its δ allotrope, which has a
The physical properties of iron at very high pressures and temperatures have also been studied extensively,
Some controversial experimental evidence exists for a stable
The
Melting and boiling points
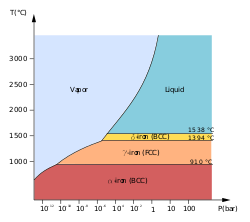
The melting and boiling points of iron, along with its enthalpy of atomization, are lower than those of the earlier 3d elements from scandium to chromium, showing the lessened contribution of the 3d electrons to metallic bonding as they are attracted more and more into the inert core by the nucleus;[15] however, they are higher than the values for the previous element manganese because that element has a half-filled 3d sub-shell and consequently its d-electrons are not easily delocalized. This same trend appears for ruthenium but not osmium.[16]
The melting point of iron is experimentally well defined for pressures less than 50 GPa. For greater pressures, published data (as of 2007) still varies by tens of gigapascals and over a thousand kelvin.[17]
Magnetic properties

Below its
In the absence of an external source of magnetic field, the atoms get spontaneously partitioned into magnetic domains, about 10 micrometers across,[20] such that the atoms in each domain have parallel spins, but some domains have other orientations. Thus a macroscopic piece of iron will have a nearly zero overall magnetic field.
Application of an external magnetic field causes the domains that are magnetized in the same general direction to grow at the expense of adjacent ones that point in other directions, reinforcing the external field. This effect is exploited in devices that need to channel magnetic fields to fulfill design function, such as
Similar behavior is exhibited by some iron compounds, such as the ferrites including the mineral magnetite, a crystalline form of the mixed iron(II,III) oxide Fe3O4 (although the atomic-scale mechanism, ferrimagnetism, is somewhat different). Pieces of magnetite with natural permanent magnetization (lodestones) provided the earliest compasses for navigation. Particles of magnetite were extensively used in magnetic recording media such as core memories, magnetic tapes, floppies, and disks, until they were replaced by cobalt-based materials.
Isotopes
Iron has four stable
60Fe is an
In phases of the meteorites Semarkona and Chervony Kut, a correlation between the concentration of 60Ni, the
The most abundant iron isotope 56Fe is of particular interest to nuclear scientists because it represents the most common endpoint of
Although a further tiny energy gain could be extracted by synthesizing 62Ni, which has a marginally higher binding energy than 56Fe, conditions in stars are unsuitable for this process. Element production in supernovas greatly favor iron over nickel, and in any case, 56Fe still has a lower mass per nucleon than 62Ni due to its higher fraction of lighter protons.[29] Hence, elements heavier than iron require a supernova for their formation, involving rapid neutron capture by starting 56Fe nuclei.[26]
In the far future of the universe, assuming that proton decay does not occur, cold fusion occurring via quantum tunnelling would cause the light nuclei in ordinary matter to fuse into 56Fe nuclei. Fission and alpha-particle emission would then make heavy nuclei decay into iron, converting all stellar-mass objects to cold spheres of pure iron.[30]
Origin and occurrence in nature
Cosmogenesis
Iron's abundance in rocky planets like Earth is due to its abundant production during the runaway fusion and explosion of type Ia supernovae, which scatters the iron into space.[31][32]
Metallic iron
Metallic or
The rare
Mantle minerals
Ferropericlase (Mg,Fe)O, a solid solution of periclase (MgO) and wüstite (FeO), makes up about 20% of the volume of the lower mantle of the Earth, which makes it the second most abundant mineral phase in that region after silicate perovskite (Mg,Fe)SiO3; it also is the major host for iron in the lower mantle.[36] At the bottom of the transition zone of the mantle, the reaction γ-(Mg,Fe)2[SiO4] ↔ (Mg,Fe)[SiO3] + (Mg,Fe)O transforms γ-olivine into a mixture of silicate perovskite and ferropericlase and vice versa. In the literature, this mineral phase of the lower mantle is also often called magnesiowüstite.[37] Silicate perovskite may form up to 93% of the lower mantle,[38] and the magnesium iron form, (Mg,Fe)SiO3, is considered to be the most abundant mineral in the Earth, making up 38% of its volume.[39]
Earth's crust

While iron is the most abundant element on Earth, most of this iron is concentrated in the inner and outer cores.[40][41] The fraction of iron that is in Earth's crust only amounts to about 5% of the overall mass of the crust and is thus only the fourth most abundant element in that layer (after oxygen, silicon, and aluminium).[42]
Most of the iron in the crust is combined with various other elements to form many iron minerals. An important class is the iron oxide minerals such as hematite (Fe2O3), magnetite (Fe3O4), and siderite (FeCO3), which are the major ores of iron. Many igneous rocks also contain the sulfide minerals pyrrhotite and pentlandite.[43][44] During weathering, iron tends to leach from sulfide deposits as the sulfate and from silicate deposits as the bicarbonate. Both of these are oxidized in aqueous solution and precipitate in even mildly elevated pH as iron(III) oxide.[45]

Large deposits of iron are
Materials containing finely ground iron(III) oxides or oxide-hydroxides, such as ochre, have been used as yellow, red, and brown pigments since pre-historical times. They contribute as well to the color of various rocks and clays, including entire geological formations like the Painted Hills in Oregon and the Buntsandstein ("colored sandstone", British Bunter).[48] Through Eisensandstein (a jurassic 'iron sandstone', e.g. from Donzdorf in Germany)[49] and Bath stone in the UK, iron compounds are responsible for the yellowish color of many historical buildings and sculptures.[50] The proverbial red color of the surface of Mars is derived from an iron oxide-rich regolith.[51]
Significant amounts of iron occur in the iron sulfide mineral pyrite (FeS2), but it is difficult to extract iron from it and it is therefore not exploited.[52] In fact, iron is so common that production generally focuses only on ores with very high quantities of it.[53]
According to the International Resource Panel's Metal Stocks in Society report, the global stock of iron in use in society is 2,200 kg per capita. More-developed countries differ in this respect from less-developed countries (7,000–14,000 vs 2,000 kg per capita).[54]
Oceans
Ocean science demonstrated the role of the iron in the ancient seas in both marine biota and climate.[55]
Chemistry and compounds
| Oxidation state |
Representative compound |
|---|---|
| −4 (d10s2) | [FeIn6−xSnx][56] |
| −2 (d10) | Disodium tetracarbonylferrate (Collman's reagent) |
| −1 (d9) | Fe 2(CO)2− 8 |
| 0 (d8) | Iron pentacarbonyl |
| 1 (d7) | Cyclopentadienyliron dicarbonyl dimer ("Fp2") |
| 2 (d6) | Ferrous sulfate, Ferrocene
|
| 3 (d5) | Ferric chloride, Ferrocenium tetrafluoroborate
|
| 4 (d4) | Fe(diars) 2Cl2+ 2, FeO(BF4)2 |
| 5 (d3) | FeO3− 4 |
| 6 (d2) | Potassium ferrate |
| 7 (d1) | [FeO4]– (matrix isolation, 4K) |
Iron shows the characteristic chemical properties of the transition metals, namely the ability to form variable oxidation states differing by steps of one and a very large coordination and organometallic chemistry: indeed, it was the discovery of an iron compound, ferrocene, that revolutionalized the latter field in the 1950s.[57] Iron is sometimes considered as a prototype for the entire block of transition metals, due to its abundance and the immense role it has played in the technological progress of humanity.[58] Its 26 electrons are arranged in the configuration [Ar]3d64s2, of which the 3d and 4s electrons are relatively close in energy, and thus a number of electrons can be ionized.[16]
Iron forms compounds mainly in the
Iron is the first of the transition metals that cannot reach its group oxidation state of +8, although its heavier congeners ruthenium and osmium can, with ruthenium having more difficulty than osmium.
Unlike many other metals, iron does not form amalgams with mercury. As a result, mercury is traded in standardized 76 pound flasks (34 kg) made of iron.[64]
Iron is by far the most reactive element in its group; it is pyrophoric when finely divided and dissolves easily in dilute acids, giving Fe2+. However, it does not react with concentrated nitric acid and other oxidizing acids due to the formation of an impervious oxide layer, which can nevertheless react with hydrochloric acid.[10] High-purity iron, called electrolytic iron, is considered to be resistant to rust, due to its oxide layer.
Binary compounds
Oxides and sulfides
Iron forms various
2 ions in a distorted sodium chloride structure.[65]
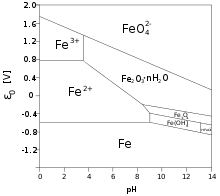
Halides

The binary ferrous and ferric
- Fe + 2 HX → FeX2 + H2 (X = F, Cl, Br, I)
Iron reacts with fluorine, chlorine, and bromine to give the corresponding ferric halides,
- 2 Fe + 3 X2 → 2 FeX3 (X = F, Cl, Br)
Ferric iodide is an exception, being thermodynamically unstable due to the oxidizing power of Fe3+ and the high reducing power of I−:[66]
- 2 I− + 2 Fe3+ → I2 + 2 Fe2+ (E0 = +0.23 V)
Ferric iodide, a black solid, is not stable in ordinary conditions, but can be prepared through the reaction of iron pentacarbonyl with iodine and carbon monoxide in the presence of hexane and light at the temperature of −20 °C, with oxygen and water excluded.[66]Complexes of ferric iodide with some soft bases are known to be stable compounds.[67][68]
Solution chemistry

The
[Fe(H2O)6]2+ + 2 e− ⇌ Fe E0 = −0.447 V [Fe(H2O)6]3+ + e− ⇌ [Fe(H2O)6]2+ E0 = +0.77 V FeO2−
4 + 8 H3O+ + 3 e−⇌ [Fe(H2O)6]3+ + 6 H2O E0 = +2.20 V
The red-purple tetrahedral ferrate(VI) anion is such a strong oxidizing agent that it oxidizes ammonia to nitrogen (N2) and water to oxygen[66]
- 4 FeO2−
4 + 34 H
2O → 4 [Fe(H2O)6]3+ + 20 OH−
+ 3 O2
The pale-violet hex
[Fe(H2O)6]3+ ⇌ [Fe(H2O)5(OH)]2+ + H+ K = 10−3.05 mol dm−3 [Fe(H2O)5(OH)]2+ ⇌ [Fe(H2O)4(OH)2]+ + H+ K = 10−3.26 mol dm−3 2[Fe(H2O)6]3+ ⇌ [Fe(H2O)4(OH)]4+2 + 2H+ + 2H2O K = 10−2.91 mol dm−3

As pH rises above 0 the above yellow hydrolyzed species form and as it rises above 2–3, reddish-brown hydrous iron(III) oxide precipitates out of solution. Although Fe3+ has a d5 configuration, its absorption spectrum is not like that of Mn2+ with its weak, spin-forbidden d–d bands, because Fe3+ has higher positive charge and is more polarizing, lowering the energy of its ligand-to-metal charge transfer absorptions. Thus, all the above complexes are rather strongly colored, with the single exception of the hexaquo ion – and even that has a spectrum dominated by charge transfer in the near ultraviolet region.[69] On the other hand, the pale green iron(II) hexaquo ion [Fe(H2O)6]2+ does not undergo appreciable hydrolysis. Carbon dioxide is not evolved when carbonate anions are added, which instead results in white iron(II) carbonate being precipitated out. In excess carbon dioxide this forms the slightly soluble bicarbonate, which occurs commonly in groundwater, but it oxidises quickly in air to form iron(III) oxide that accounts for the brown deposits present in a sizeable number of streams.[70]
Coordination compounds
Due to its electronic structure, iron has a very large coordination and organometallic chemistry.

Many coordination compounds of iron are known. A typical six-coordinate anion is hexachloroferrate(III), [FeCl6]3−, found in the mixed


Iron(III) complexes are quite similar to those of chromium(III) with the exception of iron(III)'s preference for O-donor instead of N-donor ligands. The latter tend to be rather more unstable than iron(II) complexes and often dissociate in water. Many Fe–O complexes show intense colors and are used as tests for phenols or enols. For example, in the ferric chloride test, used to determine the presence of phenols, iron(III) chloride reacts with a phenol to form a deep violet complex:[69]
- 3 ArOH + FeCl3 → Fe(OAr)3 + 3 HCl (Ar = aryl)
Among the halide and pseudohalide complexes, fluoro complexes of iron(III) are the most stable, with the colorless [FeF5(H2O)]2− being the most stable in aqueous solution. Chloro complexes are less stable and favor tetrahedral coordination as in [FeCl4]−; [FeBr4]− and [FeI4]− are reduced easily to iron(II). Thiocyanate is a common test for the presence of iron(III) as it forms the blood-red [Fe(SCN)(H2O)5]2+. Like manganese(II), most iron(III) complexes are high-spin, the exceptions being those with ligands that are high in the spectrochemical series such as cyanide. An example of a low-spin iron(III) complex is [Fe(CN)6]3−. Iron shows a great variety of electronic spin states, including every possible spin quantum number value for a d-block element from 0 (diamagnetic) to 5⁄2 (5 unpaired electrons). This value is always half the number of unpaired electrons. Complexes with zero to two unpaired electrons are considered low-spin and those with four or five are considered high-spin.[65]
Iron(II) complexes are less stable than iron(III) complexes but the preference for O-donor ligands is less marked, so that for example [Fe(NH3)6]2+ is known while [Fe(NH3)6]3+ is not. They have a tendency to be oxidized to iron(III) but this can be moderated by low pH and the specific ligands used.[70]
Organometallic compounds

carbonyl

Prussian blue or "ferric ferrocyanide", Fe4[Fe(CN)6]3, is an old and well-known iron-cyanide complex, extensively used as pigment and in several other applications. Its formation can be used as a simple wet chemistry test to distinguish between aqueous solutions of Fe2+ and Fe3+ as they react (respectively) with potassium ferricyanide and potassium ferrocyanide to form Prussian blue.[61]
Another old example of an organoiron compound is iron pentacarbonyl, Fe(CO)5, in which a neutral iron atom is bound to the carbon atoms of five carbon monoxide molecules. The compound can be used to make carbonyl iron powder, a highly reactive form of metallic iron. Thermolysis of iron pentacarbonyl gives triiron dodecacarbonyl, Fe3(CO)12, a complex with a cluster of three iron atoms at its core. Collman's reagent, disodium tetracarbonylferrate, is a useful reagent for organic chemistry; it contains iron in the −2 oxidation state. Cyclopentadienyliron dicarbonyl dimer contains iron in the rare +1 oxidation state.[76]
A landmark in this field was the discovery in 1951 of the remarkably stable sandwich compound ferrocene Fe(C5H5)2, by Pauson and Kealy[77] and independently by Miller and colleagues,[78] whose surprising molecular structure was determined only a year later by Woodward and Wilkinson[79] and Fischer.[80] Ferrocene is still one of the most important tools and models in this class.[81]
Iron-centered organometallic species are used as
Industrial uses
The iron compounds produced on the largest scale in industry are
History
Development of iron metallurgy
Iron is one of the elements undoubtedly known to the ancient world.[83] It has been worked, or wrought, for millennia. However, iron artefacts of great age are much rarer than objects made of gold or silver due to the ease with which iron corrodes.[84] The technology developed slowly, and even after the discovery of smelting it took many centuries for iron to replace bronze as the metal of choice for tools and weapons.
Meteoritic iron

Beads made from
Meteoric iron was highly regarded due to its origin in the heavens and was often used to forge weapons and tools.
Meteoritic iron is comparably soft and ductile and easily
Wrought iron

The first iron production started in the
Artifacts of smelted iron are found in
Some archaeological evidence suggests iron was smelted in Zimbabwe and southeast Africa as early as the eighth century BC.[96] Iron working was introduced to Greece in the late 11th century BC, from which it spread quickly throughout Europe.[97]

The spread of ironworking in Central and Western Europe is associated with Celtic expansion. According to Pliny the Elder, iron use was common in the Roman era.[85] In the lands of what is now considered China, iron appears approximately 700–500 BC.[98] Iron smelting may have been introduced into China through Central Asia.[99] The earliest evidence of the use of a blast furnace in China dates to the 1st century AD,[100] and cupola furnaces were used as early as the Warring States period (403–221 BC).[101] Usage of the blast and cupola furnace remained widespread during the Tang and Song dynasties.[102]
During the Industrial Revolution in Britain, Henry Cort began refining iron from pig iron to wrought iron (or bar iron) using innovative production systems. In 1783 he patented the puddling process for refining iron ore. It was later improved by others, including Joseph Hall.[103]
Cast iron

Medieval
In 1709, Abraham Darby I established a coke-fired blast furnace to produce cast iron, replacing charcoal, although continuing to use blast furnaces. The ensuing availability of inexpensive iron was one of the factors leading to the Industrial Revolution. Toward the end of the 18th century, cast iron began to replace wrought iron for certain purposes, because it was cheaper. Carbon content in iron was not implicated as the reason for the differences in properties of wrought iron, cast iron, and steel until the 18th century.[90]
Since iron was becoming cheaper and more plentiful, it also became a major structural material following the building of the innovative
Steel
Steel (with smaller carbon content than pig iron but more than wrought iron) was first produced in antiquity by using a
New methods of producing it by carburizing bars of iron in the cementation process were devised in the 17th century. In the Industrial Revolution, new methods of producing bar iron without charcoal were devised and these were later applied to produce steel. In the late 1850s, Henry Bessemer invented a new steelmaking process, involving blowing air through molten pig iron, to produce mild steel. This made steel much more economical, thereby leading to wrought iron no longer being produced in large quantities.[111]
Foundations of modern chemistry
In 1774, Antoine Lavoisier used the reaction of water steam with metallic iron inside an incandescent iron tube to produce hydrogen in his experiments leading to the demonstration of the conservation of mass, which was instrumental in changing chemistry from a qualitative science to a quantitative one.[112]
Symbolic role

Iron plays a certain role in mythology and has found various usage
The Virtues, in despair, quit the earth; and the depravity of man becomes universal and complete. Hard steel succeeded then.
— Ovid, Metamorphoses, Book I, Iron age, line 160 ff
An example of the importance of iron's symbolic role may be found in the
Production of metallic iron

Laboratory routes
For a few limited purposes when it is needed, pure iron is produced in the laboratory in small quantities by reducing the pure oxide or hydroxide with hydrogen, or forming iron pentacarbonyl and heating it to 250 °C so that it decomposes to form pure iron powder.[45] Another method is electrolysis of ferrous chloride onto an iron cathode.[115]
Main industrial route
| Country | Iron ore | Pig iron | Direct iron | Steel |
|---|---|---|---|---|
| 1,114.9 | 549.4 | 573.6 | ||
| 393.9 | 4.4 | 5.2 | ||
| 305.0 | 25.1 | 0.011 | 26.5 | |
| 66.9 | 87.5 | |||
| 257.4 | 38.2 | 23.4 | 63.5 | |
| 92.1 | 43.9 | 4.7 | 60.0 | |
| 65.8 | 25.7 | 29.9 | ||
| 0.1 | 27.3 | 48.6 | ||
| 0.4 | 20.1 | 0.38 | 32.7 | |
| World | 1,594.9 | 914.0 | 64.5 | 1,232.4 |
Nowadays, the industrial production of iron or steel consists of two main stages. In the first stage, iron ore is reduced with coke in a blast furnace, and the molten metal is separated from gross impurities such as silicate minerals. This stage yields an alloy – pig iron – that contains relatively large amounts of carbon. In the second stage, the amount of carbon in the pig iron is lowered by oxidation to yield wrought iron, steel, or cast iron.[117] Other metals can be added at this stage to form alloy steels.
Blast furnace processing
The blast furnace is loaded with iron ores, usually hematite Fe2O3 or magnetite Fe3O4, along with coke (coal that has been separately baked to remove volatile components) and flux (limestone or dolomite). "Blasts" of air pre-heated to 900 °C (sometimes with oxygen enrichment) is blown through the mixture, in sufficient amount to turn the carbon into carbon monoxide:[117]
- 2 C + O2 → 2 CO
This reaction raises the temperature to about 2000 °C. The carbon monoxide reduces the iron ore to metallic iron[117]
- Fe2O3 + 3 CO → 2 Fe + 3 CO2
Some iron in the high-temperature lower region of the furnace reacts directly with the coke:[117]
- 2 Fe2O3 + 3 C → 4 Fe + 3 CO2
The flux removes silicaceous minerals in the ore, which would otherwise clog the furnace: The heat of the furnace decomposes the carbonates to
Steelmaking thus remains one of the largest industrial contributors of CO2 emissions in the world.[118]
-
17th century Chinese illustration of workers at a blast furnace, making wrought iron from pig iron[119]
-
How iron was extracted in the 19th century
-
Iron furnace in Columbus, Ohio, 1922
Steelmaking
The pig iron produced by the blast furnace process contains up to 4–5% carbon (by mass), with small amounts of other impurities like sulfur, magnesium, phosphorus, and manganese. This high level of carbon makes it relatively weak and brittle. Reducing the amount of carbon to 0.002–2.1% produces
Steel products often undergo various
-
This heap of iron ore pellets will be used in steel production.
-
A pot of molten iron being used to make steel
Direct iron reduction
Owing to environmental concerns, alternative methods of processing iron have been developed. "
Natural gas is partially oxidized (with heat and a catalyst):[106]
- 2 CH4 + O2 → 2 CO + 4 H2
Iron ore is then treated with these gases in a furnace, producing solid sponge iron:[106]
- Fe2O3 + CO + 2 H2 → 2 Fe + CO2 + 2 H2O
Thermite process
Ignition of a mixture of aluminium powder and iron oxide yields metallic iron via the
- Fe2O3 + 2 Al → 2 Fe + Al2O3
Alternatively pig iron may be made into steel (with up to about 2% carbon) or wrought iron (commercially pure iron). Various processes have been used for this, including
Molten oxide electrolysis
Molten oxide electrolysis (MOE) uses electrolysis of molten iron oxide to yield metallic iron. It is studied in laboratory-scale experiments and is proposed as a method for industrial iron production that has no direct emissions of carbon dioxide. It uses a liquid iron cathode, an anode formed from an alloy of chromium, aluminium and iron,[121] and the electrolyte is a mixture of molten metal oxides into which iron ore is dissolved. The current keeps the electrolyte molten and reduces the iron oxide. Oxygen gas is produced in addition to liquid iron. The only carbon dioxide emissions come from any fossil fuel-generated electricity used to heat and reduce the metal.[122][123][124]
Applications
| Material | TS (MPa) |
BH (Brinell) |
|---|---|---|
| Iron whiskers | 11000 | |
| Ausformed (hardened) steel |
2930 | 850–1200 |
| Martensitic steel | 2070 | 600 |
| Bainitic steel | 1380 | 400 |
| Pearlitic steel | 1200 | 350 |
Cold-worked iron
|
690 | 200 |
| Small-grain iron | 340 | 100 |
| Carbon-containing iron | 140 | 40 |
| Pure, single-crystal iron | 10 | 3 |
As structural material
Iron is the most widely used of all the metals, accounting for over 90% of worldwide metal production. Its low cost and high strength often make it the material of choice to withstand stress or transmit forces, such as the construction of machinery and
Mechanical properties
The mechanical properties of iron and its alloys are extremely relevant to their structural applications. Those properties can be evaluated in various ways, including the Brinell test, the Rockwell test and the Vickers hardness test.
The properties of pure iron are often used to calibrate measurements or to compare tests.[126][128] However, the mechanical properties of iron are significantly affected by the sample's purity: pure, single crystals of iron are actually softer than aluminium,[125] and the purest industrially produced iron (99.99%) has a hardness of 20–30 Brinell.[129] The pure iron (99.9%~99.999%), especially called electrolytic iron, is industrially produced by electrolytic refining.
An increase in the carbon content will cause a significant increase in the hardness and tensile strength of iron. Maximum hardness of 65 Rc is achieved with a 0.6% carbon content, although the alloy has low tensile strength.[130] Because of the softness of iron, it is much easier to work with than its heavier congeners ruthenium and osmium.[16]
Types of steels and alloys

α-Iron is a fairly soft metal that can dissolve only a small concentration of carbon (no more than 0.021% by mass at 910 °C).[131] Austenite (γ-iron) is similarly soft and metallic but can dissolve considerably more carbon (as much as 2.04% by mass at 1146 °C). This form of iron is used in the type of stainless steel used for making cutlery, and hospital and food-service equipment.[20]
Commercially available iron is classified based on purity and the abundance of additives. Pig iron has 3.5–4.5% carbon[132] and contains varying amounts of contaminants such as sulfur, silicon and phosphorus. Pig iron is not a saleable product, but rather an intermediate step in the production of cast iron and steel. The reduction of contaminants in pig iron that negatively affect material properties, such as sulfur and phosphorus, yields cast iron containing 2–4% carbon, 1–6% silicon, and small amounts of manganese.[117] Pig iron has a melting point in the range of 1420–1470 K, which is lower than either of its two main components, and makes it the first product to be melted when carbon and iron are heated together.[10] Its mechanical properties vary greatly and depend on the form the carbon takes in the alloy.[16]
"White" cast irons contain their carbon in the form of cementite, or iron carbide (Fe3C).[16] This hard, brittle compound dominates the mechanical properties of white cast irons, rendering them hard, but unresistant to shock. The broken surface of a white cast iron is full of fine facets of the broken iron carbide, a very pale, silvery, shiny material, hence the appellation. Cooling a mixture of iron with 0.8% carbon slowly below 723 °C to room temperature results in separate, alternating layers of cementite and α-iron, which is soft and malleable and is called pearlite for its appearance. Rapid cooling, on the other hand, does not allow time for this separation and creates hard and brittle martensite. The steel can then be tempered by reheating to a temperature in between, changing the proportions of pearlite and martensite. The end product below 0.8% carbon content is a pearlite-αFe mixture, and that above 0.8% carbon content is a pearlite-cementite mixture.[16]
In gray iron the carbon exists as separate, fine flakes of graphite, and also renders the material brittle due to the sharp edged flakes of graphite that produce stress concentration sites within the material.[133] A newer variant of gray iron, referred to as ductile iron, is specially treated with trace amounts of magnesium to alter the shape of graphite to spheroids, or nodules, reducing the stress concentrations and vastly increasing the toughness and strength of the material.[133]
Alloys with high purity elemental makeups (such as alloys of
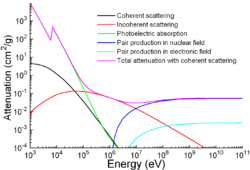
Apart from traditional applications, iron is also used for protection from ionizing radiation. Although it is lighter than another traditional protection material, lead, it is much stronger mechanically. The attenuation of radiation as a function of energy is shown in the graph.[137]
The main disadvantage of iron and steel is that pure iron, and most of its alloys, suffer badly from rust if not protected in some way, a cost amounting to over 1% of the world's economy.[138] Painting, galvanization, passivation, plastic coating and bluing are all used to protect iron from rust by excluding water and oxygen or by cathodic protection. The mechanism of the rusting of iron is as follows:[138]
- Cathode: 3 O2 + 6 H2O + 12 e− → 12 OH−
- Anode: 4 Fe → 4 Fe2+ + 8 e−; 4 Fe2+ → 4 Fe3+ + 4 e−
- Overall: 4 Fe + 3 O2 + 6 H2O → 4 Fe3+ + 12 OH− → 4 Fe(OH)3 or 4 FeO(OH) + 4 H2O
The electrolyte is usually iron(II) sulfate in urban areas (formed when atmospheric sulfur dioxide attacks iron), and salt particles in the atmosphere in seaside areas.[138]
Catalysts and reagents
Because Fe is inexpensive and nontoxic, much effort has been devoted to the development of Fe-based catalysts and reagents. Iron is however less common as a catalyst in commercial processes than more expensive metals.[139] In biology, Fe-containing enzymes are pervasive.[140]
Iron catalysts are traditionally used in the
Iron compounds
Biological and pathological role
Iron is required for life.[9][146][147] The iron–sulfur clusters are pervasive and include nitrogenase, the enzymes responsible for biological nitrogen fixation. Iron-containing proteins participate in transport, storage and use of oxygen.[9] Iron proteins are involved in electron transfer.[148]

Examples of iron-containing proteins in higher organisms include hemoglobin, cytochrome (see high-valent iron), and catalase.[9][149] The average adult human contains about 0.005% body weight of iron, or about four grams, of which three quarters is in hemoglobin – a level that remains constant despite only about one milligram of iron being absorbed each day,[148] because the human body recycles its hemoglobin for the iron content.[150]
Microbial growth may be assisted by oxidation of iron(II) or by reduction of iron (III).[151]
Biochemistry
Iron acquisition poses a problem for aerobic organisms because ferric iron is poorly soluble near neutral pH. Thus, these organisms have developed means to absorb iron as complexes, sometimes taking up ferrous iron before oxidising it back to ferric iron.[9] In particular, bacteria have evolved very high-affinity sequestering agents called siderophores.[152][153][154]
After uptake in human
The most commonly known and studied
Hemoglobin is an oxygen carrier that occurs in red blood cells and contributes their color, transporting oxygen in the arteries from the lungs to the muscles where it is transferred to myoglobin, which stores it until it is needed for the metabolic oxidation of glucose, generating energy.[9] Here the hemoglobin binds to carbon dioxide, produced when glucose is oxidized, which is transported through the veins by hemoglobin (predominantly as bicarbonate anions) back to the lungs where it is exhaled.[148] In hemoglobin, the iron is in one of four heme groups and has six possible coordination sites; four are occupied by nitrogen atoms in a porphyrin ring, the fifth by an imidazole nitrogen in a histidine residue of one of the protein chains attached to the heme group, and the sixth is reserved for the oxygen molecule it can reversibly bind to.[148] When hemoglobin is not attached to oxygen (and is then called deoxyhemoglobin), the Fe2+ ion at the center of the heme group (in the hydrophobic protein interior) is in a high-spin configuration. It is thus too large to fit inside the porphyrin ring, which bends instead into a dome with the Fe2+ ion about 55 picometers above it. In this configuration, the sixth coordination site reserved for the oxygen is blocked by another histidine residue.[148]
When deoxyhemoglobin picks up an oxygen molecule, this histidine residue moves away and returns once the oxygen is securely attached to form a hydrogen bond with it. This results in the Fe2+ ion switching to a low-spin configuration, resulting in a 20% decrease in ionic radius so that now it can fit into the porphyrin ring, which becomes planar.[148] (Additionally, this hydrogen bonding results in the tilting of the oxygen molecule, resulting in a Fe–O–O bond angle of around 120° that avoids the formation of Fe–O–Fe or Fe–O2–Fe bridges that would lead to electron transfer, the oxidation of Fe2+ to Fe3+, and the destruction of hemoglobin.) This results in a movement of all the protein chains that leads to the other subunits of hemoglobin changing shape to a form with larger oxygen affinity. Thus, when deoxyhemoglobin takes up oxygen, its affinity for more oxygen increases, and vice versa.[148] Myoglobin, on the other hand, contains only one heme group and hence this cooperative effect cannot occur. Thus, while hemoglobin is almost saturated with oxygen in the high partial pressures of oxygen found in the lungs, its affinity for oxygen is much lower than that of myoglobin, which oxygenates even at low partial pressures of oxygen found in muscle tissue.[148] As described by the Bohr effect (named after Christian Bohr, the father of Niels Bohr), the oxygen affinity of hemoglobin diminishes in the presence of carbon dioxide.[148]
- 4 Cytc2+ + O2 + 8H+
inside → 4 Cytc3+ + 2 H2O + 4H+
outside
Although the heme proteins are the most important class of iron-containing proteins, the
The ability of sea
Nutrition
Diet
Iron is pervasive, but particularly rich sources of dietary iron include
Iron provided by dietary supplements is often found as iron(II) fumarate, although iron(II) sulfate is cheaper and is absorbed equally well.[143] Elemental iron, or reduced iron, despite being absorbed at only one-third to two-thirds the efficiency (relative to iron sulfate),[163] is often added to foods such as breakfast cereals or enriched wheat flour. Iron is most available to the body when chelated to amino acids[164] and is also available for use as a common iron supplement. Glycine, the least expensive amino acid, is most often used to produce iron glycinate supplements.[165]
Dietary recommendations
The U.S. Institute of Medicine (IOM) updated Estimated Average Requirements (EARs) and Recommended Dietary Allowances (RDAs) for iron in 2001.
The European Food Safety Authority (EFSA) refers to the collective set of information as Dietary Reference Values, with Population Reference Intake (PRI) instead of RDA, and Average Requirement instead of EAR. AI and UL defined the same as in United States. For women the PRI is 13 mg/day ages 15–17 years, 16 mg/day for women ages 18 and up who are premenopausal and 11 mg/day postmenopausal. For pregnancy and lactation, 16 mg/day. For men the PRI is 11 mg/day ages 15 and older. For children ages 1 to 14 the PRI increases from 7 to 11 mg/day. The PRIs are higher than the U.S. RDAs, with the exception of pregnancy.[167] The EFSA reviewed the same safety question did not establish a UL.[168]
Infants may require iron supplements if they are bottle-fed cow's milk.[169] Frequent blood donors are at risk of low iron levels and are often advised to supplement their iron intake.[170]
For U.S. food and dietary supplement labeling purposes the amount in a serving is expressed as a percent of Daily Value (%DV). For iron labeling purposes 100% of the Daily Value was 18 mg, and as of May 27, 2016[update] remained unchanged at 18 mg.[171][172] A table of the old and new adult daily values is provided at Reference Daily Intake.
Deficiency
Iron deficiency is the most common
The brain is resistant to acute iron deficiency due to the slow transport of iron through the blood brain barrier.[178] Acute fluctuations in iron status (marked by serum ferritin levels) do not reflect brain iron status, but prolonged nutritional iron deficiency is suspected to reduce brain iron concentrations over time.[179][180] In the brain, iron plays a role in oxygen transport, myelin synthesis, mitochondrial respiration, and as a cofactor for neurotransmitter synthesis and metabolism.[181] Animal models of nutritional iron deficiency report biomolecular changes resembling those seen in Parkinson's and Huntington's disease.[182][183] However, age-related accumulation of iron in the brain has also been linked to the development of Parkinson's.[184]
Excess
Overdoses of ingested iron can cause excessive levels of free iron in the blood. High blood levels of free ferrous iron react with
The medical management of iron toxicity is complicated, and can include use of a specific chelating agent called deferoxamine to bind and expel excess iron from the body.[188][191][192]
ADHD
Some research has suggested that low thalamic iron levels may play a role in the pathophysiology of ADHD.[193] Some researchers have found that iron supplementation can be effective especially in the inattentive subtype of the disorder.[194]
Some researchers in the 2000s suggested a link between low levels of iron in the blood and ADHD. A 2012 study found no such correlation.[195]
Cancer
The role of iron in cancer defense can be described as a "double-edged sword" because of its pervasive presence in non-pathological processes.
Marine systems
Iron plays an essential role in marine systems and can act as a limiting nutrient for planktonic activity.[198] Because of this, too much of a decrease in iron may lead to a decrease in growth rates in phytoplanktonic organisms such as diatoms.[199] Iron can also be oxidized by marine microbes under conditions that are high in iron and low in oxygen.[200]
Iron can enter marine systems through adjoining rivers and directly from the atmosphere. Once iron enters the ocean, it can be distributed throughout the water column through ocean mixing and through recycling on the cellular level.[201] In the arctic, sea ice plays a major role in the store and distribution of iron in the ocean, depleting oceanic iron as it freezes in the winter and releasing it back into the water when thawing occurs in the summer.[202] The iron cycle can fluctuate the forms of iron from aqueous to particle forms altering the availability of iron to primary producers.[203] Increased light and warmth increases the amount of iron that is in forms that are usable by primary producers.[204]
See also
- Economically important iron deposits include:
- Carajás Minein the state of Pará, Brazil, is thought to be the largest iron deposit in the world.
- El Mutún in Bolivia, where 10% of the world's accessible iron ore is located.
- Hamersley Basin is the largest iron ore deposit in Australia.
- Kiirunavaara in Sweden, where one of the world's largest deposits of iron ore is located
- The Mesabi Iron Range is the chief iron ore mining district in the United States.
- Iron and steel industry
- Iron cycle
- Iron nanoparticle
- Iron–platinum nanoparticle
- Iron fertilization – proposed fertilization of oceans to stimulate phytoplankton growth
- Iron-oxidizing bacteria
- List of countries by iron production
- Pelletising – process of creation of iron ore pellets
- Rustproof iron
- Steel
References
- ^ "Standard Atomic Weights: Iron". CIAAW. 1993.
- ISSN 1365-3075.
- .
- .
- PMID 27812577.
- ISBN 978-1-62708-155-9.
- ISBN 1-84628-668-9.
- .
- ^ a b c d e f g h i j k l m n o p q "Iron". Micronutrient Information Center, Linus Pauling Institute, Oregon State University, Corvallis, Oregon. April 2016. Retrieved 6 March 2018.
- ^ a b c d e f g h Greenwood & Earnshaw 1997, pp. 1075–79.
- S2CID 206528628.
- .
- S2CID 33458168.
- .
- ^ Greenwood & Earnshaw 1997, p. 1116.
- ^ a b c d e f Greenwood & Earnshaw 1997, pp. 1074–75.
- ISBN 9780444527486.
- ^ Steinmetz, Charles (1917). "fig. 42". Theory and Calculation of Electric Circuits. McGraw-Hill.
- ^ ISBN 978-0-471-47741-9.
- ^ ISBN 978-0-87170-748-2.
- ^
- PMID 19792637.
- PMID 16463281. Archived from the original(PDF) on 10 June 2010.
- .
- doi:10.1119/1.17828.
- ^ a b c Greenwood & Earnshaw 1997, p. 12.
- S2CID 118974639.
- S2CID 14437704.
- Bibcode:1995AAS...186.3707B.
- .
- ^ Aron, Jacob. "Supernova space bullets could have seeded Earth's iron core". New Scientist. Retrieved 2 October 2020.
- ^ Croswell, Ken. "Iron in the Fire: The Little-Star Supernovae That Could". Scientific American. Retrieved 3 January 2021.
- .
- ISBN 978-0-521-40949-0.
- .
- ^ Stark, Anne M. (20 September 2007) Researchers locate mantle's spin transition zone, leading to clues about earth's structure. Lawrence Livermore National Laboratory
- ^ Ferropericlase. Mindat.org
- S2CID 4387193.
- S2CID 206563252.
- S2CID 121861429.
- .
- PMID 16592930.
- ^ "Pyrrhotite". Mindat.org. Retrieved 7 July 2009.
- ISBN 0-471-80580-7
- ^ a b Greenwood & Earnshaw 1997, p. 1071.
- S2CID 205049360.
- .
- ^ Dickinson, Robert E. (1964). Germany: A regional and economic geography (2nd ed.). London: Methuen.
- ^ Naturwerksteine in Baden-Württemberg. Landesamt für Geologie, Rohstoffe und Bergbau, Baden-Württemberg
- ^ "Tales From The Riverbank". Minerva Stone Conservation. Archived from the original on 28 September 2015. Retrieved 22 September 2015.
- S2CID 98227499.
- OCLC 913701506.
- OCLC 855858511.
- UNEP
- S2CID 211139074.
- .
- ^ Greenwood & Earnshaw 1997, p. 905.
- ^ a b Greenwood & Earnshaw 1997, p. 1070.
- PMID 27812577.
- PMID 17469792. Archived from the original(PDF) on 15 June 2021. Retrieved 22 February 2022.
- ^ ISBN 3-11-007511-3.
- ISBN 978-0-306-41647-7.
- ISBN 978-1-900747-07-3.
- ^ Gmelin, Leopold (1852). "Mercury and Iron". Hand-book of chemistry. Vol. 6. Cavendish Society. pp. 128–29.
- ^ a b c Greenwood & Earnshaw 1997, p. 1079.
- ^ a b c d Greenwood & Earnshaw 1997, pp. 1082–84.
- ^ Siegfried Pohl, Ulrich Bierbach, Wolfgang Saak; "FeI3SC(NMe2)2, a Neutral Thiourea Complex of Iron(III) Iodide", Angewandte Chemie International Edition in English (1989) 28 (6), 776-777. https://doi.org/10.1002/anie.198907761
- ^ Nicholas A. Barnes, Stephen M.Godfrey, Nicholas Ho, Charles A.McAuliffe, Robin G.Pritchard; "Facile synthesis of a rare example of an iron(III) iodide complex, [FeI3(AsMe3)2], from the reaction of Me3AsI2 with unactivated iron powder", Polyhedron (2013) 55, 67-72. https://doi.org/10.1016/j.poly.2013.02.066
- ^ a b c d Greenwood & Earnshaw 1997, pp. 1088–91.
- ^ a b Greenwood & Earnshaw 1997, pp. 1091–97.
- .
- .
- ISBN 978-0-470-13248-7.
- S2CID 35665289.
- S2CID 98739882.
- ISBN 978-0-08-022057-4..
- S2CID 4181383.
- .
- .
- ISSN 1434-1948.
- ^ Greenwood & Earnshaw 1997, p. 1104.
- PMID 17847139.
- ^ Weeks 1968, p. 4.
- ^ a b Weeks 1968, p. 29.
- ^ a b c Weeks 1968, p. 31.
- .
- .
- ^ Walsh, Declan (2 June 2016). "King Tut's Dagger Made of 'Iron From the Sky,' Researchers Say". The New York Times. Archived from the original on 3 January 2022. Retrieved 4 June 2016.
the blade's composition of iron, nickel and cobalt was an approximate match for a meteorite that landed in northern Egypt. The result "strongly suggests an extraterrestrial origin"
- ^ Ure, Andrew (1843). Technisches wörterbuch oder Handbuch der Gewerbskunde ... : Bearb. nach Dr. Andrew Ure's Dictionary of arts, manufactures and mines (in German). G. Haase. p. 492.
- ^ a b c d Weeks 1968, p. 32.
- ^ McNutt, Paula (1990 1). The Forging of Israel: Iron Technology, Symbolism and Tradition in Ancient Society. A&C Black.
- ^ Tewari, Rakesh. "The origins of Iron Working in India: New evidence from the Central Ganga plain and the Eastern Vindhyas" (PDF). State Archaeological Department. Retrieved 23 May 2010.
- JSTOR 124562.
- ^ Muhly, James D. (2003). "Metalworking/Mining in the Levant". In Lake, Richard Winona (ed.). Near Eastern Archaeology IN: Eisenbrauns. Vol. 180. pp. 174–83.
- ^ Witzel, Michael (2001), "Autochthonous Aryans? The Evidence from Old Indian and Iranian Texts", in Electronic Journal of Vedic Studies (EJVS) 7-3, pp. 1–93
- ^ Weeks, p. 33, quoting Cline, Walter (1937) "Mining and Metallurgy in Negro Africa", George Banta Publishing Co., Menasha, Wis., pp. 17–23.
- Brill's New Pauly, Brill.
- ISBN 0-465-00304-4. p. 10.
- ISBN 0-924171-34-0, p. 8.
- ISBN 978-0-521-58000-7.
earliest blast furnace discovered in China from about the first century AD
- ISBN 0-924171-34-0, p. 191.
- ^ The Coming of the Ages of Steel. Brill Archive. 1961. p. 54.
- .
- .
- ISBN 90-04-16549-5, p. 200.
- ^ a b c d e f g Biddle, Verne; Parker, Gregory. Chemistry, Precision and Design. A Beka Book, Inc.
- ISBN 978-90-04-09632-5.
- ^ a b c d Greenwood & Earnshaw 1997, p. 1072.
- ^ Schivelbusch, G. (1986) The Railway Journey: Industrialization and Perception of Time and Space in the 19th Century. Oxford: Berg.
- ^ Spoerl, Joseph S. A Brief History of Iron and Steel Production Archived 2 June 2010 at the Wayback Machine. Saint Anselm College
- ISBN 978-3-527-61234-5.
- .
- S2CID 161808359.
- ISBN 3-7861-1130-8
- ^ Lux, H. (1963) "Metallic Iron" in Handbook of Preparative Inorganic Chemistry, 2nd Ed. G. Brauer (ed.), Academic Press, NY. Vol. 2. pp. 1490–91.
- ^ Steel Statistical Yearbook 2010. World Steel Association
- ^ a b c d e f g Greenwood & Earnshaw 1997, p. 1073.
- PMID 33824307.
- ^ Song Yingxing (1637): The Tiangong Kaiwu encyclopedia.
- ^ Verhoeven, J.D. (1975) Fundamentals of Physical Metallurgy, Wiley, New York, p. 326
- ISSN 0028-0836.
- ISSN 0021-891X.
- .
- ^ Gallucci, Maria (7 September 2023). "Boston Metal gets big funding boost to make green steel". Canary Media. Rocky Mountain Institute. Retrieved 11 March 2024.
- ^ ISBN 1-56396-387-6.
- ^ ISBN 0-87170-389-0. Archived from the original(PDF) on 9 February 2019. Retrieved 22 February 2022.
- ^ Greenwood & Earnshaw 1997, pp. 1070–71.
- ^ "Hardness Conversion Chart". Maryland Metrics. Archived from the original on 18 June 2015. Retrieved 23 May 2010.
- .
- ISBN 81-203-2455-2.
- ISBN 978-0-08-045127-5.
- ^ ISBN 1-147-64423-3.
- ^ ISBN 0-07-295358-6.
- ^ a b "Classification of Carbon and Low-Alloy Steels". Archived from the original on 2 January 2011. Retrieved 5 January 2008.
- ^ "HSLA Steel". 15 November 2002. Archived from the original on 30 December 2009. Retrieved 11 October 2008.
- Bibcode:1984msh..book.....R.
- ^ Rokni, Sayed H.; Cossairt, J. Donald; Liu, James C. (January 2008). "Radiation Shielding at High-Energy Electron and Proton Accelerators" (PDF). Retrieved 6 August 2016.
- ^ a b c Greenwood & Earnshaw 1997, p. 1076.
- PMID 27981231.
- PMID 32792370.
- ISBN 978-0-471-49244-3.
- ISBN 978-0-8247-2481-8.
- ^ ISBN 3-527-30673-0.
- ISBN 978-1-4465-4656-7.
- hdl:10665/345533. WHO/MHP/HPS/EML/2021.02.
- ISBN 978-94-007-5561-1
- ^
Yee, Gereon M.; Tolman, William B. (2015). "Transition Metal Complexes and the Activation of Dioxygen". In Peter M.H. Kroneck; Martha E. Sosa Torres (eds.). Sustaining Life on Planet Earth: Metalloenzymes Mastering Dioxygen and Other Chewy Gases. Metal Ions in Life Sciences. Vol. 15. Springer. pp. 131–204. PMID 25707468.
- ^ a b c d e f g h i j k l m n o p Greenwood & Earnshaw 1997, pp. 1098–104.
- ISBN 0-935702-73-3.
- PMID 16115609.
- S2CID 196704759.
- PMID 7592901.
- PMID 6455965.
- S2CID 19697776.
- PMID 16224057.
- PMID 14691550.
- ^ Boon EM, Downs A, Marcey D. "Proposed Mechanism of Catalase". Catalase: H2O2: H2O2 Oxidoreductase: Catalase Structural Tutorial. Retrieved 11 February 2007.
- PMID 8502991.
- PMID 8070415.
- PMID 9761903.
- . Retrieved 2 November 2017.
- ^ Food Standards Agency – Eat well, be well – Iron deficiency Archived 8 August 2006 at the Wayback Machine. Eatwell.gov.uk (5 March 2012). Retrieved on 27 June 2012.
- S2CID 42983904.
- PMID 11377130.
- ISBN 0-87983-501-X.
- PMID 25057538. Archived from the original(PDF) on 9 September 2017. Retrieved 9 March 2017.
- ^ "Overview on Dietary Reference Values for the EU population as derived by the EFSA Panel on Dietetic Products, Nutrition and Allergies" (PDF). European Food Safety Authority. 2017.
- ^ "Tolerable Upper Intake Levels For Vitamins And Minerals" (PDF). European Food Safety Authority. 2006.
- ^ "Iron Deficiency Anemia". MediResource. Archived from the original on 16 December 2008. Retrieved 17 December 2008.
- PMID 8867722.
- ^ "Federal Register May 27, 2016 Food Labeling: Revision of the Nutrition and Supplement Facts Labels. FR page 33982" (PDF).
- ^ "Daily Value Reference of the Dietary Supplement Label Database (DSLD)". Dietary Supplement Label Database (DSLD). Archived from the original on 7 April 2020. Retrieved 16 May 2020.
- PMID 12418542.
- PMID 24470094.
- ISBN 978-94-007-5561-1
- ^ CDC Centers for Disease Control and Prevention (3 April 1998). "Recommendations to Prevent and Control Iron Deficiency in the United States". Morbidity and Mortality Weekly Report. 47 (RR3): 1. Retrieved 12 August 2014.
- ^ Centers for Disease Control and Prevention. "Iron and Iron Deficiency". Retrieved 12 August 2014.
- PMID 2773840.
- PMID 9311961.
- PMID 24999026.
- PMID 25231526.
- PMID 28567002.
- PMID 12672939.
- PMID 26962053.
- ISBN 978-0-7216-7335-6. Retrieved 27 June 2012.
- S2CID 28909635.
- PMID 11109593.
- ^ PMID 7837315.
- ^ a b "Toxicity, Iron". Medscape. Retrieved 23 May 2010.
- ^ Dietary Reference Intakes (DRIs): Recommended Intakes for Individuals (PDF), Food and Nutrition Board, Institute of Medicine, National Academies, 2004, archived from the original (PDF) on 14 March 2013, retrieved 9 June 2009
- PMID 8800185.
- PMID 21102602.
- PMID 32992575.
- PMID 23582950.
- S2CID 22445593.
- PMID 29394034.
- ^ PMID 24275533.
- ^ Lannuzel, D., Vancoppenolle, M., van der Merwe, P., de Jong, J., Meiners, K.M., Grotti, M., Nishioska, J., & Schoemann. (2016). Iron in sea ice: Review and new insights. Elementa: Science of the Anthropocene, 4 000130.
- ^ Tagliabue, A., Bopp, L., Aumont, O., & Arrigo, K.R. (2009). Influence of light and temperature on the marine iron cycle: From theoretical to global modeling. Global Biogeochemical Cycles, 23.
Bibliography
- ISBN 978-0-08-037941-8.
- LCCN 68-15217.
Further reading
- H.R. Schubert, History of the British Iron and Steel Industry ... to 1775 AD (Routledge, London, 1957)
- R.F. Tylecote, History of Metallurgy (Institute of Materials, London 1992).
- R.F. Tylecote, "Iron in the Industrial Revolution" in J. Day and R.F. Tylecote, The Industrial Revolution in Metals (Institute of Materials 1991), 200–60.
External links
- It's Elemental – Iron
- Iron at The Periodic Table of Videos(University of Nottingham)
- Metallurgy for the non-Metallurgist
- Iron by J. B. Calvert






![17th century Chinese illustration of workers at a blast furnace, making wrought iron from pig iron[119]](http://upload.wikimedia.org/wikipedia/commons/thumb/8/8c/Chinese_Fining_and_Blast_Furnace.jpg/191px-Chinese_Fining_and_Blast_Furnace.jpg)



