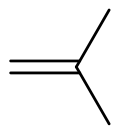
Isobutylene
| |||
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
2-Methylprop-1-ene | |||
| Other names
2-Methylpropene
Isobutene γ-Butylene 2-Methylpropylene Methylpropene | |||
| Identifiers | |||
3D model (
JSmol ) |
|||
| ChEBI | |||
| ChemSpider | |||
ECHA InfoCard
|
100.003.697 | ||
| EC Number |
| ||
PubChem CID
|
|||
RTECS number
|
| ||
| UNII | |||
| UN number | 1075
| ||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties[1] | |||
| C4H8 | |||
| Molar mass | 56.106 g/mol | ||
| Appearance | Colorless gas | ||
| Density | 0.5879 g/cm3, liquid | ||
| Melting point | −140.3 °C (−220.5 °F; 132.8 K) | ||
| Boiling point | −6.9 °C (19.6 °F; 266.2 K) | ||
| -44.4·10−6 cm3/mol | |||
| Hazards[2] | |||
| GHS labelling: | |||
 
| |||
| Danger | |||
| H220 | |||
| P210, P377, P381, P403 | |||
| NFPA 704 (fire diamond) | |||
| Flash point | flammable gas | ||
| 465 °C (869 °F; 738 K) | |||
Explosive limits
|
1.8–9.6% | ||
| Related compounds | |||
Related butenes
|
trans-2-Butene
| ||
Related compounds
|
Isobutane | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Isobutylene (or 2-methylpropene) is a
isomers of butylene. It is a colorless flammable gas, and is of considerable industrial value.[3]
Production
Polymer and chemical grade isobutylene is typically obtained by dehydrating
tertiary butyl alcohol (TBA) or catalytic dehydrogenation of isobutane (Catofin or similar processes).[4] Gasoline additives methyl tert-butyl ether (MTBE) and ethyl tert-butyl ether (ETBE), respectively, are produced by reacting methanol or ethanol
with isobutylene contained in butene streams from olefin steam crackers or refineries, or with isobutylene from dehydrated TBA. Isobutylene is not isolated from the olefin or refinery butene stream before the reaction, as separating the ethers from the remaining butenes is simpler. Isobutylene can also be produced in high purities by "back-cracking" MTBE or ETBE at high temperatures and then separating the isobutylene by distillation from methanol.
Isobutylene is a byproduct in the ethenolysis of diisobutylene to prepare neohexene:[5]
- (CH3)3C-CH=C(CH3)2 + CH2=CH2 → (CH3)3C-CH=CH2 + (CH3)2C=CH2
Uses
Isobutylene is used in the production of a variety of products. It is alkylated with butane to produce
Friedel-Crafts alkylation of phenols
with isobutylene.
catalysts:[6]
- NH3 + CH2=C(CH3)2 → H2NC(CH3)3
Applications are found in the calibration of photoionization detectors.
Safety
Isobutylene is a highly flammable gas.
See also
- Butyl rubber
- Polythene
- Polybutene
- Perfluoroisobutene
References
- ISBN 091191028X, 5024.
- ^ Isobutene, International Chemical Safety Card 1027, Geneva: International Programme on Chemical Safety, April 2000
- ISBN 978-3527306732.
- ISBN 978-0-471-41782-8.
- ^ Lionel Delaude; Alfred F. Noels. "Metathesis". Kirk-Othmer Encyclopedia of Chemical Technology. Wiley.
- ISBN 978-3527306732.
External links
- International Chemical Safety Card 1027
- SIDS Initial Assessment Report for Isobutylene from the Organisation for Economic Co-operation and Development(OECD)



