Isopentenyl-diphosphate delta isomerase
| isopentenyl-diphosphate Δ-isomerase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
ExPASy NiceZyme view | | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| Gene Ontology | AmiGO / QuickGO | ||||||||
| |||||||||
| Isopentenyl-pyrophosphate Δ isomerase 1 | |||||||
|---|---|---|---|---|---|---|---|
| Identifiers | |||||||
| Symbol | IDI1 | ||||||
Chr. 10 p15.3 | |||||||
| |||||||
Isopentenyl pyrophosphate isomerase (
- isopentenyl diphosphate dimethylallyl diphosphate
This enzyme belongs to the family of isomerases, specifically those intramolecular oxidoreductases transposing C=C bonds. The systematic name of this enzyme class is isopentenyl-diphosphate Delta3-Delta2-isomerase. Other names in common use include isopentenylpyrophosphate Delta-isomerase, methylbutenylpyrophosphate isomerase, and isopentenylpyrophosphate isomerase.[2][3][4]
Enzyme mechanism
IPP isomerase catalyzes the isomerization of IPP to DMAPP by an antarafacial transposition of hydrogen.[5][6] The empirical evidence suggests that this reaction proceeds by a protonation/deprotonation mechanism, with the addition of a proton to the re-face of the inactivated C3-C4 double bond resulting in a transient carbocation intermediate.[7][8] The removal of the pro-R proton from C2 forms the C2-C3 double bond of DMAPP.
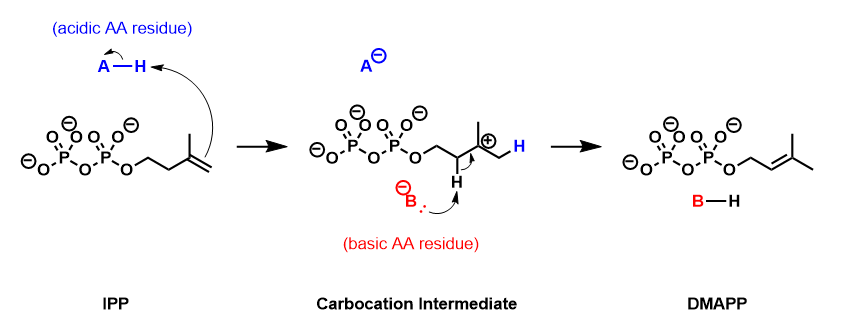
Enzyme structure

IPP isomerase also requires a
The coordination of the metal cation to the glutamate residue stabilizes the carbiocation intermediate after protonation.Structural studies
As of late 2007, 25
Biological function
The protonation of an inactivated double bond is rarely seen in nature, highlighting the unique catalytic mechanism of IPP isomerase. The isomerization of IPP to DMAPP is a crucial step in the synthesis of isoprenoids and isoprenoid-derivatives, compounds that play vital roles in the biosynthetic pathways of all living organisms.

Disease relevance
References
- ^ "IDI1 - Isopentenyl-diphosphate Delta-isomerase - Saccharomyces cerevisiae (strain ATCC 204508 / S288c) (Baker's yeast) - IDI1 gene & protein". UniProt. Retrieved 6 June 2016.
- PMID 11158573.
- PMID 6351725.
- PMID 13792054.
- PMID 4288360.
- ^ Cornforth RH, Popják G (1969). "Chemical syntheses of substrates of sterol biosynthesis". In Raymond BC (ed.). Methods in Enzymology. Vol. 15. Academic Press. pp. 359–390.
- PMID 3022798.
- .
- ^ PMID 1285217.
- PMID 17250851.
- PMID 7908830.
- PMID 12540835.
- PMID 17137593.
- PMID 11698677.
- S2CID 3999323.
- PMID 9418296.
- S2CID 19430360.
- S2CID 1637634.
- PMID 18303110.
- PMID 20955688.
External links
- isopentenyldiphosphate+delta-isomerase at the U.S. National Library of Medicine Medical Subject Headings (MeSH)

