Itraconazole
 | |
| Clinical data | |
|---|---|
| Trade names | Sporanox, Sporaz, Orungal, others |
| Other names | ITZ |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a692049 |
| License data |
|
| Pregnancy category |
|
vaginal suppository, intravenous | |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | ~55%, maximal if taken with full meal |
| Protein binding | 99.8% |
| Metabolism | Extensive in liver (CYP3A4) |
| Metabolites | Hydroxy-itraconazole, keto-itraconazole, N-desalkyl-itraconazole[4] |
| Elimination half-life | 21 hours |
| Excretion | Kidney (35%), faeces (54%)[5] |
| Identifiers | |
| |
JSmol) | |
| Chirality | Racemic mixture |
| Melting point | 165[6] °C (329 °F) |
| Solubility in water | 7.8 ± 0.4 × 10−6 mol/L (pH 1.6)[6] mg/mL (20 °C) |
| |
| |
| (verify) | |
Itraconazole, sometimes abbreviated ITZ, is an
Common
Itraconazole was patented in 1978 and approved for medical use in the United States in 1992.[7][8] It is on the World Health Organization's List of Essential Medicines.[9]
Recent research works suggest itraconazole (ITZ) could also be used in the treatment of cancer by inhibiting the
.Medical uses
Itraconazole has a broader spectrum of activity than fluconazole (but not as broad as voriconazole or posaconazole). In particular, it is active against Aspergillus, which fluconazole is not. It is also licensed for use in blastomycosis, sporotrichosis, histoplasmosis, and onychomycosis. Itraconazole is over 99% protein-bound and has virtually no penetration into cerebrospinal fluid. Therefore, it should not be used to treat meningitis or other central nervous system infections.[11] According to the Johns Hopkins Abx Guide, it has "negligible CSF penetration, however treatment has been successful for cryptococcal and coccidioidal meningitis".[12]
It is also prescribed for systemic infections, such as
Itraconazole has been explored as an anticancer agent for patients with
Available forms
Itraconazole is produced as blue 22 mm (0.87 in) capsules with tiny 1.5 mm (0.059 in) blue pellets inside. Each capsule contains 100 mg and is usually taken twice a day at twelve-hour intervals. The Sporanox brand of itraconazole has been developed and marketed by
The oral solution is better absorbed. The
Side effects
Itraconazole is a relatively well-tolerated drug (although not as well tolerated as fluconazole or voriconazole) and the range of adverse effects it produces is similar to the other azole antifungals:[21]
- elevated alanine aminotransferaselevels are found in 4% of people taking itraconazole
- "small but real risk" of developing congestive heart failure[21]
- liver failure, sometimes fatal
The cyclodextrin used to make the syrup preparation can cause diarrhea. Side effects that may indicate a greater problem include:[citation needed]
Interactions
The following drugs should not be taken with itraconazole:[22]
- amiodarone (Cordarone);[23]
- cisapride
- dofetilide
- nisoldipine
- alcohol
- pimozide
- quinidine
- lurasidone
- lovastatin or simvastatin
- midazolam or triazolam
- ergot medicines such as
- dihydroergotamine
- ergometrine
- ergotamine
- methylergonovine
Pharmacology
Pharmacodynamics
The mechanism of action of itraconazole is the same as the other azole antifungals: it inhibits the fungal-mediated synthesis of ergosterol, via inhibition of lanosterol 14α-demethylase. Because of its ability to inhibit cytochrome P450 3A4 CC-3, caution should be used when considering interactions with other medications.[24]
Itraconazole is pharmacologically distinct from other
Pharmacokinetics
Itraconazole, like
A product (Lozanoc) licensed through the European union decentralised procedure[34] has increased bioavailability, decreased sensitivity to co ingestion of food, and hence decreased variability of serum levels.
Chemistry

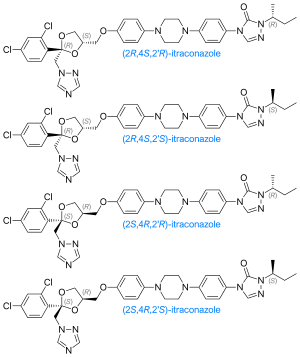
The itraconazole molecule has three chiral carbons. The two chiral centers in the dioxolane ring are fixed in relation to one another, and the triazolomethylene and aryloxymethylene dioxolane-ring substituents are always cis to each other. The clinical formulation is a 1:1:1:1 mixture of four stereoisomers (two enantiomeric pairs).[35][36]
History
Itraconazole was approved for medical use in the United States in 1992.[37]
It was designated an orphan drug by both the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA).[38][39][40][41][42]
References
- ^ a b "Itraconazole Use During Pregnancy". Drugs.com. 20 March 2019. Retrieved 15 May 2020.
- FDA. Retrieved 22 October 2023.
- ^ "Sporanox 10 mg/mL Oral Solution - Summary of Product Characteristics (SmPC)". (emc). 1 February 2018. Retrieved 15 May 2020.
- S2CID 6941636.
- ^ "Sporanox (itraconazole) Capsules. Full Prescribing Information" (PDF). Janssen Pharmaceuticals, Inc. Archived from the original (PDF) on 17 May 2018. Retrieved 28 August 2016.
- ^ S2CID 232135660.
- ^ a b c d e f g h "Itraconazole". The American Society of Health-System Pharmacists. Retrieved 8 December 2017.
- ISBN 9783527607495.
- hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- PMID 31692536.
- ISBN 978-1-930808-30-0.[page needed]
- ^ Pham P, Bartlett JG (24 July 2007). "Itraconazole". Johns Hopkins. Archived from the original on 28 November 2007.
- U.S. National Institutes of Health.
- PMID 21896639.
- PMID 23340005.
- PMID 23546045.
- S2CID 235608763.
- ^ a b US 20050226924, Lee KH, Park ES, Chi SC, "Composition comprising Itraconazole for oral administration", published 13 October 2005, assigned to FDL Inc.
- ^ "Sporanox (Itraconazole Capsules)" (PDF). Janssen. June 2006. Archived from the original (PDF) on 5 July 2008.
- ^ "SUBA Bioavailability Technology". Mayne Pharma Group.
- ^ a b "The Safety of Sporanox Capsules and Lamisil Tablets for the Treatment of Onychomycosis". FDA Public Health Advisory. 9 May 2001. Archived from the original on 28 May 2009. Retrieved 10 August 2006.
- ^ "Sporanox (Itraconazole) Capsules". Safety Labeling Changes Approved By FDA Center for Drug Evaluation and Research. U.S. Food and Drug Administration (FDA). Archived from the original on 26 October 2016. Retrieved 16 December 2019.
- PMID 21801420.
- ISBN 978-0-07-182635-8.
- PMID 20385363.
- PMID 23291299.
- ^ PMID 17432820.
- ^ PMID 21896639.
- PMID 20176935.
- PMID 21936514.
- PMID 8878612.
- PMID 9477653.
- PMID 8388198.
- ^ "Lozanoc 50 Mg Hard Capsules (Itraconazole)" (PDF). Public Assessment Report Decentralised Procedure. UK Medicines and Health Care Products Regulatory Agency.
- S2CID 189994.
- ^ "Itraconazole on Drugs.com". Drugs.com. Retrieved 28 August 2016.
- ^ "Itraconazole: FDA-Approved Drugs". U.S. Food and Drug Administration (FDA). Retrieved 15 May 2020.
- ^ "Itraconazole Orphan Drug Designation". U.S. Food and Drug Administration (FDA). 19 May 2016. Retrieved 15 May 2020.
- ^ "Itraconazole Orphan Drug Designation". U.S. Food and Drug Administration (FDA). 16 August 2016. Retrieved 15 May 2020.
- ^ "Itraconazole Orphan Drug Designation". U.S. Food and Drug Administration (FDA). 30 October 2008. Retrieved 15 May 2020.
- ^ "EU/3/17/1901". European Medicines Agency (EMA). 23 August 2017. Retrieved 15 May 2020.
- ^ "EU/3/18/2024". European Medicines Agency (EMA). 25 May 2018. Retrieved 15 May 2020.
External links
 Media related to Itraconazole at Wikimedia Commons
Media related to Itraconazole at Wikimedia Commons
