Kwashiorkor
| Kwashiorkor | |
|---|---|
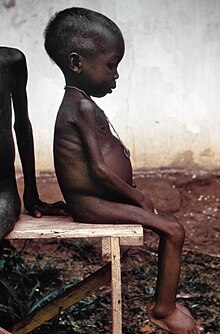 | |
| A young girl with kwashiorkor in a relief camp during the Biafra War | |
| Specialty | Pediatrics |
Kwashiorkor (
Conditions analogous to kwashiorkor were well documented around the world throughout history.
Classification
Kwashiorkor is a type of severe acute malnutrition (SAM). SAM is a category, composed of two conditions: marasmus and kwashiorkor.[9] Both kwashiorkor and marasmus fall under the umbrella of protein–energy malnutrition (PEM).[10] These diseases are oftentimes discussed together, but are distinctly separate conditions of malnutrition. Kwashiorkor is marked by an array of metabolic disturbances of uncertain etiology. In contrast, marasmus is more clearly a syndrome of energy deficiency, which is marked by weight loss. On physical exam, kwashiorkor is also distinguished from marasmus by the presence of edema. When children present with both kwashiorkor and marasmus, the condition is referred to as "marasmic-kwashiorkor".[11][3] In general, kwashiorkor is marked by more profound serum depletions of antioxidant molecules and minerals, relative to marasmus.[3]
Wellcome's classification
Wellcome classification[12] is a system for classifying protein-energy malnutrition in children based on weight for their age and based on presence of edema. Other classifications include Gomez classification and Waterlow classification.[13][14]
| Weight for age | With edema | Without edema | General considerations |
|---|---|---|---|
| 60-80% | Kwashiorkor | Undernutrition |
|
| <60% | Marasmic kwashiorkor | Marasmus |
Signs and symptoms
The defining sign of kwashiorkor in children is bilateral
Causes
The precise etiology of kwashiorkor remains unclear.[15][16][17][18] Several hypotheses have been proposed that are associated with and explain some, but not all aspects of the pathophysiology of kwashiorkor. They include, but are not limited to protein deficiency causing hypoalbuminemia, amino acid deficiency, oxidative stress, and gut microbiome changes.[15][18][19]
Low protein intake

Kwashiorkor is a severe form of
Extreme fluid retention observed in individuals suffering from kwashiorkor is accompanied by irregularities in the lymphatic system as well as disruptions of capillary exchange. The lymphatic system serves three major purposes: fluid recovery, immunity, and lipid absorption. Victims of kwashiorkor commonly exhibit reduced ability to recover fluids, immune system failure, and low lipid absorption. Fluid recovery by the lymphatic system is accomplished by re-vascularization of fluid and macromolecules from the interstitial space, allowing these constituents of whole blood to be returned to the venous circulation. Compromised fluid recovery may contribute to the phenomenon of extravascular fluid accumulation in kwashiorkor.[21]
The low protein theory for the pathogenesis of kwashiorkor has been used to teach that capillary exchange between the lymphatic system and circulating blood is impaired by a reduced oncotic (i.e. colloid osmotic pressure, COP) in the blood, as a consequence of inadequate protein intake, so that the hydrostatic pressure gradient, which favors extravasation of fluid from small vessels, is not overcome. Proteins, mainly albumin, are responsible for creating the COP observed in the blood and tissue fluids. The difference in the COP of the blood and tissue tends to favor the reentry of fluid from the extravascular space, into the circulatory system. This tendency is opposed by the venous hydrostatic pressure, which tends to favor the exit of fluid from small vessels, into the interstitial space. The low protein theory for the pathogenesis of kwashiorkor held that a deficiency of serum proteins, caused by inadequate protein intake, disrupted this balance, and thus impaired the return flow of fluid from the interstitium into the capillary and venous structures. It has been taught that this is what accounts for the accumulation of extravascular fluid in kwashiorkor, and the subsequent pedal edema and abdominal distension.[22]
The low protein theory, which relies heavily upon Starling's theory for the movement of fluid in biological systems, provided a compelling rationale for the pathogenesis of edema in kwashiorkor. What it does not explain however, is the entire array of disturbances that define the kwashiorkor syndrome. These include, irritability, anorexia, skin desquamation, skin depigmentation, hair discoloration, reduced mitochondrial respiration, impaired lipid export from the liver without an accompanying reduction of lipoprotein synthesis, 'oxidative stress', glutathione depletions,
Social factors are also relevant. Ignorance of nutrition can be a cause. A case was described where parents who fed their child cassava failed to recognize malnutrition because of the edema caused by the syndrome and believed the child was well-nourished despite the development of kwashiorkor.[24]
Aflatoxins
Recent studies have attempted to pinpoint a relationship between kwashiorkor and high levels of aflatoxins. Aflatoxins are naturally occurring toxins produced by the mold Aspergillus flavus, a fungus found in areas with hot and humid climates.[25] These toxins tend to grow and can be found in agricultural crops such as millet, maize, and rice.[25] An analysis found that the presence of aflatoxins was found more frequently and in higher concentrations in individuals with kwashiorkor when compared to individuals with marasmus (another form of severe acute malnutrition).[26][27] In particular, biological samples showed greater levels of aflatoxins in the brain, heart, kidney, liver, lungs, serum, stool, and urine.[26] Aflatoxins were not found in liver samples of individuals with marasmus.[26] It has been known that the liver organ is the main target of aflatoxins and chronic toxicity can result in immunosuppressive and carcinogenic effects.[26] However, there is currently conflicting evidence to pinpoint a connection between kwashiorkor and aflatoxins. Studies have shown that not all children with kwashiorkor present with detectable aflatoxin levels.[3] It has also been proposed that damage done by aflatoxins may be due to glutathione depletion (another proposed mechanism of the disease) in children with kwashiorkor.[3]
Mechanisms
Peripheral edema and hypoalbuminemia
Kwashiorkor is a form of protein deficiency, which can result in both osmotic imbalances and irregularities in the lymphatic system.[3]
Kwashiorkor is most notable for peripheral edema. The presence of edema in kwashiorkor is correlated with very low albumin concentration (hypoalbuminemia). Edema results from a loss of fluid balance between the hydrostatic and oncotic pressures across the capillary blood vessel walls[2] due to the lack of protein which affects the body's ability to draw fluid from the tissues into the bloodstream. Low albumin concentration influences negatively the strength of oncotic pressure. Failure leads to the fluid buildup in the abdomen, resulting in edema and belly distension.[3]
Furthermore, the release of antidiuretic hormone is stimulated by hypovolemia, also leading to the development of peripheral edema. Plasma renin is also stimulated, promoting sodium retention.[2]
It is important to distinguish the pathophysiology of marasmus and kwashiorkor when it comes to treating malnourished children who may have hypovolemic shock that is cause by an acute loss of salt and water.[16] Children with severe albumin deficiency struggle physiologically to maintain their blood volume.[16]
Low glutathione levels
Kwashiorkor is also marked by low glutathione levels. Glutathione is used in many of the body processes on a molecule level.[28]
It is believed to be related to high oxidant levels commonly seen in people who suffer from starvation and rarely in chronic inflammation.[2] Glutathione serves vital functions including management of oxidative stress which is an imbalance that plays a key role in the pathogenesis of many diseases.
Evidence indicates that amino acid balance has an important effect on protein nutrition and therefore on glutathione homeostasis.[29]
Cysteine is an essential amino acid that acts as the limiting amino acid for glutathione synthesis in humans. Factors that increase demand for glutathione may increase demand for cysteine, and hence methionine. Such demands have been hypothesized to increase risk for kwashiorkor.
Others
A proposed experimental theory suggests that alterations in the microbiome/virone contributes to edematous malnutrition, but further studies are required to understand the mechanism.[2]
Diagnosis
Kwashiorkor, or edematous malnutrition, like many other malnutrition diseases, is indirectly assessed using anthropometry.[9] Kwashiorkor is a subtype of severe acute malnutrition (SAM) characterized by bilateral peripheral pitting edema. According to the World Health Organization, the SAM diagnosis parameters are a "mid-upper arm circumference (MUAC) of < 115 mm, weight-for-height/length Z-score (WHZ) of < -3Z and nutritional edema or any combination of these parameters."[30][2][31] Additional clinical findings on physical exam include marked muscle atrophy, abdominal distension, dermatitis, and hepatomegaly.[2][32]
WHO criteria for clinical assessment of malnutrition are based on the degree of wasting (MUAC), stunting (weight-for-height Z-score), and the presence of edema (mild to severe).[33]
Screening
Because it can be difficult to measure weight-for-height Z scores (WHZ) frequently, screening is performed by physical exam, with careful examination of the child's feet to detect the presence of bilateral pitting edema. Screening for edema is essential for the diagnosis of kwashiorkor, since nearly two thirds of kwashiorkor cases do not have evidence of acute wasting (i.e. mid-upper arm circumference (MUAC) < 125 mm, or WHZ < -2) when diagnosed with kwashiorkor.
Prevention
As for the prevention of childhood malnutrition, there needs to be public health changes such as improving agriculture and improving access to healthcare to effectively reduce the rates of malnutrition in children. By educating individuals of childbearing age on proper nutrition and health during and after pregnancy, they can provide their children with the appropriate nutrients from a young age. By ensuring they are equipped with the proper education and resources, caretakers and infants are in better health, ultimately preventing childhood malnutrition.[9]
Because edema can hide decreased muscle mass, it can be hard to diagnose kwashiorkor in young children; however, if cases are overlooked, children become more susceptible to infections and can ultimately lead to morbidity and mortality.[34] To prevent this from happening, parents can be educated on proper nutrition and the importance of breastfeeding infants to ensure they receive all the nutrients they need.[34]
A diet rich in carbohydrates, fats that make up 10% of the total caloric needs, and proteins that make up 15% of the caloric needs can prevent kwashiorkor.
Proteins can be found in the following foods
- Seafood
- Peas
- Nuts
- Seeds
- Eggs
- Lean meat
- Beans[3]
Treatment
- Treat/prevent hypoglycemia
- Treat/prevent hypothermia
- Treat/prevent dehydration
- Correct electrolyte imbalance
- Treat/prevent infection
- Correct micronutrient deficiencies
- Start cautious feeding
- Achieve catch-up growth
- Provide sensory stimulation and emotional support
- Prepare for follow-up after recovery
Both clinical subtypes of severe acute malnutrition (kwashiorkor and marasmus) are treated similarly.[18][33] Upon initial treatment, children with kwashiorkor may experience weight loss as their edema resolves.[36] Therefore, after concerns of refeeding syndrome have passed, children may require 120-140% of their estimated caloric needs in order to achieve catch-up growth.[36]
The cause, type, and severity of malnutrition determines what type of treatment would be most appropriate.[37] For primary acute malnutrition, children with no complications are treated at home and are encouraged to either continue breastfeeding (for infants) or start using ready-to-use therapeutic foods (for children).[37] For secondary acute malnutrition, the underlying cause needs to be identified to appropriately treat children. Only after the primary disease is determined can an appropriate dietary plan be made, as fluid, vitamins, and macronutrients may need to be considered to not exacerbate the cause of the malnutrition.[37]
Ready-to-use therapeutic foods (RUTFs) and F-75 and F-100 milks were created to provide appropriate nutrition and caloric intake to those experiencing malnutrition. F-75 milk would be ideal when trying to reintroduce food into a malnourished person, and F-100 milk would be used to aid in weight gain. While RUTFs and F-100 milk were made to have the same nutritional value, RUTFs are beneficial as they are dehydrated and do not require much preparation.[9]
Prognosis
Kwashiorkor is associated with a high risk of mortality and long-term complications. Treatment under the guidelines of the World Health Organization has proven to reduce this mortality risk and affected children tend to recover faster than children with other severe malnutrition diseases. However, physical and intellectual capabilities are not fully restored. Growth stunting and chronic disruption of microbiota are commonly observed after recovery.[3]
A high risk of death is identified by a brachial perimeter < 11 cm or by a weight-for-age threshold < −3 z-scores below the median of the WHO child growth standards. In practice, malnourished children with edema are suffering from potentially life-threatening severe malnutrition.[38]
Epidemiology
Kwashiorkor is rare in high income countries. It is mostly observed in low-income and middle income nations and regions such as Southeast Asia, Central America, Congo, Ethiopia, Puerto Rico, Jamaica, South Africa, and Uganda, where poverty is prominent.[3] Occurrences of severe malnutrition also tend to trend higher under conditions of food insecurity, higher prevalence of infectious diseases, lack of access to appropriate care, and poor living situations with inadequate sanitation.[9] Communities experiencing famine are affected the most especially during the rainy season. Prevalence varies, but it affects children of either sex commonly under five years old.[3][10] "Globally, kwashiorkor indirected accounted for 53% of deaths among children under five between 2000 and 2003 when associated with other common childhood diseases like acute respiratory infections, malaria, measles, HIV/AIDS and other causes of perinatal deaths."[10]
When compared to marasmus in developing countries, kwashiorkor typically has a lower prevalence, "0.2%-1.6% for kwashiorkor and 1.2%-6.8% for marasmus."[3] Factors such as "diet, geographical locations, climate and aflatoxin exposure" have been invoked as potential causes for observed differences in the prevalence for kwashiorkor and marasmus.[3]
In general, in areas where Severe Acute Malnutrition (SAM) is prevalent, marasmus is more often the dominant SAM condition. However, in certain areas kwashiorkor may be more common than marasmus.
History
Kwashiorkor was present in the world long before 1933, when Cecily Williams published research which took the
Effects on pharmacokinetics
Those experiencing poverty-related infectious diseases (PRDs) such as malaria and tuberculosis are also likely to be malnourished.[39] Malnutrition can affect the pharmacokinetics of various drugs used to treat PRDs by changing a drug's bioavailability, distribution, and elimination.[39] To optimize treatment of those diseases, there needs to be more research into how severe malnutrition, specifically kwashiorkor, can affect treatment response.[39]
Research directions
Current research and recommendations to manage severe acute malnutrition (SAM), such as kwashiorkor, in children are largely based on expert opinions. Only one-third of the WHO guidelines for management of SAM are based on epidemiological and clinical research. Further studies are needed in order to "improve treatment outcomes in the large number of children with SAM."[40]
See also
References
- ISBN 978-1-4058-8118-0.
- ^ .
- ^ from the original on 10 February 2023. Retrieved 9 February 2023.
- PMID 11346341.[permanent dead link]
- ^ PMID 34084092.
- ^ PMID 6347092.
- ^ PMID 14997245.
- (PDF) from the original on 1 December 2021. Retrieved 25 February 2022.
- ^ PMID 28933421.
- ^ PMID 20393967.
- ^ "Malnutrition (Kwashiorkor and Marasmus) — Symptoms and Treatment". The Lecturio Online Medical Library. 2017. Archived from the original on 27 October 2021. Retrieved 27 July 2021.
- ^ "Protein energy malnutrition classification - wikidoc". www.wikidoc.org. Archived from the original on 15 September 2022. Retrieved 29 July 2021.
- ISBN 978-0-19-157975-2. Archivedfrom the original on 21 July 2022. Retrieved 30 July 2021.
- PMID 10822936.
- ^ a b Briend A (2014). "Kwashiorkor: still an enigma – the search must go on" (PDF). Emergency Nutrition Network. Archived (PDF) from the original on 15 February 2022. Retrieved 2 August 2019.
- ^ PMID 25223408.
- PMID 31235833.
- ^ PMID 23363771.
- PMID 27918230.
- ^ "Mortality and Burden of Disease Estimates for WHO Member States in 2002" (xls). World Health Organization. 2002. Archived from the original on 16 January 2013. Retrieved 5 October 2020.
- S2CID 220181068.
- ISBN 978-0-07-337825-1.
- S2CID 13050691.
- ^ "Malnutrition in Third World Countries". www.religion-online.org. Archived from the original on 19 September 2015. Retrieved 2 March 2017.
- ^ PMID 28144235.
- ^ S2CID 220698925.
- S2CID 24089209.
- from the original on 19 June 2022. Retrieved 29 July 2021.
- PMID 31083508.
- hdl:1854/LU-5700347.
- PMID 30217196.
- S2CID 24731334.
- ^ ISBN 978-92-4-150632-8.[page needed]
- ^ PMID 32843945.
- ^ Ashworth A (2003). "Guidelines for the inpatient treatment of severely malnourished children" (PDF). WHO. Archived from the original (PDF) on 27 March 2006.
- ^ PMID 19931063.
- ^ PMID 32806622.
- ^ "Management of moderate malnutrition in under-5 children by the health sector" (PDF). Archived (PDF) from the original on 27 January 2018. Retrieved 6 May 2021.
- ^ S2CID 235259789.
- PMID 31670269.
External links
- Picot, J; Hartwell, D; Harris, P; Mendes, D; Clegg, A J; Takeda, A (2012). "The effectiveness of interventions to treat severe acute malnutrition in young children: a systematic review". Health Technology Assessment. 16 (19): 1–316. .
 Media related to Kwashiorkor at Wikimedia Commons
Media related to Kwashiorkor at Wikimedia Commons The dictionary definition of kwashiorkor at Wiktionary
The dictionary definition of kwashiorkor at Wiktionary
