LY-503430
(Redirected from
LY-503,430
) | |
| Clinical data | |
|---|---|
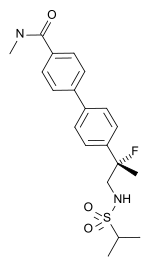
| Other names | LY-503430; (R)-4'-[1-fluoro-1-methyl-2-(propane-2-sulfonylamino)-ethyl]-biphenyl-4-carboxylic acid methylamide |
| Routes of administration | Oral |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
JSmol) | |
| |
| |
| | |
LY-503430 is an
LY-503430 produces both
BDNF in the brain, particularly in the substantia nigra, hippocampus, and striatum.[2][3] It is orally active and the main application it is currently being developed for is treatment of Parkinson's disease, although it has also been proposed to be useful in the treatment of Alzheimer's disease, depression, and schizophrenia.[4][5]
