Lurasidone
 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation | /ljʊəˈræsɪˌdoʊn/ |
| Trade names | Latuda, others |
| Other names | SM-13496 |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a611016 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth |
| Drug class | Atypical antipsychotic[2] |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 9–19% (oral)[3] |
| Protein binding | ~99%[9] |
| Metabolism | Liver (CYP3A4-mediated)[3] |
| Elimination half-life | 18–40 hours[3][9] |
| Excretion | Faecal (67–80%), renal (9–19%)[3][9] |
| Identifiers | |
| |
JSmol) | |
| Specific rotation | [α]20D −59° |
| Melting point | 176 to 178 °C (349 to 352 °F) |
| Solubility in water | 0.224 |
| |
| |
Lurasidone, sold under the brand name Latuda among others, is an
Common side effects include sleepiness,
Lurasidone was first approved for medical use in the United States in 2010.[2] In 2013, it was approved in Canada, and by the United States Food and Drug Administration, to treat bipolar depression, either as monotherapy or adjunctively with lithium or valproate.[15][16] Generic versions were approved in the United States in 2019, and became available in 2023.[17][18] In 2021, it was the 193rd most commonly prescribed medication in the United States, with more than 2 million prescriptions.[19][20]
Medical uses
Lurasidone is used to treat schizophrenia and bipolar disorder.[2][21] In bipolar disorder, It has been studied both as a monotherapy and adjunctive treatment to lithium or valproate.[22]
The European Medicines Agency approved lurasidone for the treatment of schizophrenia for people aged 13 years and older,[23] but not for bipolar disorder.[8] In the United States, it is used to treat schizophrenia for people aged 13 years and older, as well as depressive episodes of bipolar disorder age 10 and over as a monotherapy, and in conjunction with lithium or valproate in adults.[24]
In July 2013, lurasidone received approval for bipolar I depression.[25][26][27][28]
In June 2020, lurasidone was approved in Japan, eight years after its first approval in the United States.
Few available atypical antipsychotics are known to possess antidepressant efficacy in bipolar disorder (with the notable exceptions being quetiapine,[33][34][35][36] olanzapine[37][38][39] and possibly asenapine[40]) as a monotherapy, even though the majority of atypical antipsychotics are known to possess significant antimanic activity,[41] which is yet to be clearly demonstrated for lurasidone.
In the early post approval period lurasidone-treated patients with bipolar disorder were retrospectively found to have more complex clinical profiles, comorbidities, and prior treatment history compared to patients initiated with other atypical antipsychotics. The study authors suggest this may be due to "the overall clinical profile of lurasidone, the role perceived for lurasidone in the therapeutic armamentarium by practitioners, and the recent introduction of lurasidone into clinical practice during the study period."[42]
Lurasidone is not approved by the Food and Drug Administration (FDA) for the treatment of behavior disorders in older adults with dementia.[43]
Contraindications
Lurasidone is
Side effects
Side effects are generally similar to other antipsychotics. The drug has a relatively well tolerated side effect profile, with low propensity for QTc interval changes,[49][50] weight gain and lipid-related adverse effects.[51] In a 2013 meta-analysis of the efficacy and tolerability of 15 antipsychotic drugs it was found to produce the second least (after haloperidol) weight gain, the least QT interval prolongation, the fourth most extrapyramidal side effects (after haloperidol, zotepine and chlorpromazine) and the sixth least sedation (after paliperidone, sertindole, amisulpride, iloperidone and aripiprazole).[52]
As with other atypical neuroleptics, lurasidone should be used with caution in the elderly because it puts them at an increased risk for a stroke or transient ischemic attack;[53][54] however, these risks are not likely to be greater than those associated with antipsychotics of other classes.[55] Similarly, lurasidone should not be used to treat dementia-related psychosis, as evidence has shown increased mortality with antipsychotic use.[56]
Weight gain is reported in up to 15 and 16 percent of users.[57][58] Other possible side effects include vomiting, akathisia, dystonia, parkinsonism, somnolence, dizziness, sedation and nausea.[59][60]
Discontinuation
The British National Formulary recommends a gradual withdrawal when discontinuing antipsychotics to avoid acute withdrawal syndrome or rapid relapse.[61] Symptoms of withdrawal commonly include nausea, vomiting, and loss of appetite.[62] Other symptoms may include restlessness, increased sweating, and trouble sleeping.[62] Less commonly there may be a feeling of the world spinning, numbness, or muscle pains.[62] Symptoms generally resolve after a short period of time.[62]
There is tentative evidence that discontinuation of antipsychotics can result in psychosis.[63] It may also result in reoccurrence of the condition that is being treated.[64] Rarely tardive dyskinesia can occur when the medication is stopped.[62]
Interactions
Pharmacology
Pharmacodynamics
| Site | Ki (nM) | Action | Species | Ref |
|---|---|---|---|---|
| SERT | >1,000 | ND | ND | [66] |
| NET | ND | ND | ND | ND |
| DAT | >1,000 | ND | ND | [66] |
| 5-HT1A | 6.75 | Partial agonist | Rat | [66] |
| 5-HT2A | 2.03 | Antagonist | Rat | [66] |
| 5-HT2B | ND | ND | ND | ND |
| 5-HT2C | 415 | ND | Pig | [66] |
| 5-HT3 | >1,000 | ND | ND | [66] |
| 5-HT4 | >1,000 | ND | ND | [66] |
| 5-HT7 | 0.5 | Antagonist | Human | [67][66] |
| α1 | 47.9 | ND | Rat | [66] |
| α2A | 41 | ND | Human | [66] |
| α2B | ND | ND | ND | ND |
| α2C | 10.8 | Antagonist | Human | [66] |
| β1 | >1,000 | ND | ND | [66] |
| β2 | >1,000 | ND | ND | [66] |
D1 |
262 | ND | ND | [66] |
D2 |
1.68 | Antagonist | Rat | [66] |
D3 |
15.7 | Antagonist | ND | ND |
D4.4 |
30 | ND | ND | ND |
D5 |
ND | ND | ND | ND |
H1 |
>1,000 | ND | Guinea pig | [66] |
| M1 | >1,000 | ND | Human | [66] |
| Values are Ki (nM). The smaller the value, the more strongly the drug binds to the site. | ||||
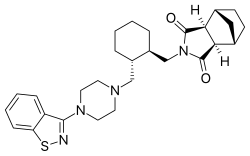
Lurasidone [(3aR,4S,7R,7aS)-2-{(1R,2R)-2-[4-(1,2-benzisothiazol-3-yl) piperazin-1-ylmethyl]-cyclohexylmethyl}-hexahydro-4,7-methano-2Hisoindole-1,3-dione hydrochloride] ]
It has only low and likely clinically unimportant
The relationship between dose and
Pharmacokinetics

Lurasidone is taken by mouth and has an estimated absorption rate of 9 to 19%.
Lurasidone is extensively metabolised by CYP3A4 leading to contraindication of both strong inhibitors as well as strong inducers of this enzyme,[82] but has negligible affinity to other cytochrome P450 enzymes. It is transported by P-glycoprotein and ABCG2 and also inhibits these carrier proteins in vitro. It also inhibits the solute carrier protein SLC22A1, but no other relevant transporters.[9][53]
Main metabolism pathways are oxidative N-
History
Lurasidone was first synthesised circa 2003.[83]
Lurasidone is a
Lurasidone is chemically similar to perospirone (also a chemical analogue of ziprasidone), as well as risperidone, paliperidone and iloperidone.[85]
It has approval from the US
Society and culture

Cost
In Canada, as of 2014, lurasidone is generally more expensive than risperidone and quetiapine but less expensive than aripiprazole.[86]
In the US, because a number of doses have the same price per tablet, pill splitting has been used to decrease costs.[87] In 2019, generic versions were approved in the United States; however, they only became available in 2023 due to drug patents.[17][18]
Brand names
In India, this drug is available under the brand names of Atlura, Lurace, Lurafic, Luramax (Sun Pharma), Lurasid, Lurastar, Latuda, Lurata[88] and additionally as Alsiva, Emsidon, Lurakem, Luratrend, Tablura, and Unison.[89]
Regulatory approval
Lurasidone was approved in the United States for the treatment of schizophrenia in October 2010[90][91] and for the treatment of depressive episodes associated with bipolar I disorder in June 2013.[25][27][28] It received regulatory approval in the United Kingdom in September 2014. In October 2014, NHS Scotland advised use of lurasidone for schizophrenic adults who have not seen improvements with previous antipsychotics due to problems that arise from weight gain or changes in metabolic pathways when taking other medications.[92] The Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) issued a positive opinion for it in January 2014, and it was approved for medical use by the EMA in March 2014.[8] It was launched in Canada for the treatment of schizophrenia in September 2012, Health Canada giving their Summary Basis of Decision (SBD) as favourable on 15 October 2012.[93] European Commission has granted a marketing authorization for once-daily oral lurasidone for the treatment of schizophrenia in adults.[94] It is approved for use in the EU.[8]
Generic versions of lurasidone were approved for use in the United States in January 2019, and became available in 2023.[95]
References
- ^ "Lurasidone (Latuda) Use During Pregnancy". Drugs.com. 5 February 2020. Archived from the original on 15 October 2020. Retrieved 12 May 2020.
- ^ a b c d e f g "Lurasidone Hydrochloride Monograph for Professionals". Drugs.com. American Society of Health-System Pharmacists. Archived from the original on 21 March 2019. Retrieved 21 March 2019.
- ^ a b c d e f g h i "Product information Latuda (lurasidone hydrochloride)" (PDF). TGA eBusiness Services. Therapeutic Goods Administration. 28 October 2022. Archived from the original on 28 October 2022. Retrieved 28 October 2022.
- ^ "Prescription medicines: registration of new chemical entities in Australia, 2014". Therapeutic Goods Administration (TGA). 21 June 2022. Archived from the original on 10 April 2023. Retrieved 10 April 2023.
- ^ Anvisa (31 March 2023). "RDC Nº 784 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial" [Collegiate Board Resolution No. 784 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Diário Oficial da União (published 4 April 2023). Archived from the original on 3 August 2023. Retrieved 16 August 2023.
- ^ "Latuda 18.5mg film-coated tablets - Summary of Product Characteristics (SmPC)". (emc). 16 January 2019. Archived from the original on 14 October 2020. Retrieved 12 May 2020.
- ^ "Latuda- lurasidone hydrochloride tablet, film coated". DailyMed. Archived from the original on 28 August 2021. Retrieved 12 May 2020.
- ^ a b c d "Latuda EPAR". European Medicines Agency (EMA). 17 September 2018. Archived from the original on 11 August 2020. Retrieved 12 May 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ a b c d e f g h i "Latuda: EPAR – Product Information" (PDF). European Medicines Agency. 14 April 2016. Archived (PDF) from the original on 21 August 2016. Retrieved 27 February 2017.
- ^ "IMPORTANT SAFETY INFORMATION AND INDICATIONS FOR LATUDA". Latuda. Archived from the original on 3 December 2022. Retrieved 3 December 2022.
- PMID 29162032.
- PMID 35609885.
- ISBN 978-0-85711-338-2.
- ^ "Lurasidone (Latuda) tablets for the treatment of schizophrenia in adults" (PDF). Archived from the original (PDF) on 27 February 2021. Retrieved 30 April 2020.
- PMID 25852975.
- PMID 28168632.
- ^ a b "Generic Latuda Availability". Drugs.com. Archived from the original on 14 August 2020. Retrieved 30 April 2020.
- ^ a b Hopkins JS (19 November 2019). "Generic-Drug Approvals Soar, But Patients Still Go Without". Wall Street Journal. Archived from the original on 27 April 2020. Retrieved 30 April 2020.
- ^ "The Top 300 of 2021". ClinCalc. Archived from the original on 15 January 2024. Retrieved 14 January 2024.
- ^ "Lurasidone - Drug Usage Statistics". ClinCalc. Retrieved 14 January 2024.
- S2CID 24653390.
- S2CID 210829608.
- ^ "Latuda (lurasidone) An overview. European Medicines Agency, 2020" (PDF). Archived (PDF) from the original on 6 October 2022. Retrieved 6 October 2022.
- ^ Canadian Agency for Drugs and Technologies in Health (2014). "Key Limitations". Lurasidone Hydrochloride (Latuda): Management of Manifestations of Schizophrenia. Canadian Agency for Drugs and Technologies in Health. Archived from the original on 28 August 2021. Retrieved 30 April 2020 – via National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ a b Lowes R (2013). "Lurasidone Approved for Bipolar Depression". Medscape. Archived from the original on 2 October 2013. Retrieved 1 October 2013.
- PMID 25852975.
- ^ a b "Latuda Supplement Approval Package 1" (PDF). U.S. Food and Drug Administration (FDA). Archived (PDF) from the original on 27 July 2020. Retrieved 12 May 2020.
- ^ a b "Latuda Supplement Approval Package 2" (PDF). U.S. Food and Drug Administration (FDA). Archived (PDF) from the original on 28 August 2021. Retrieved 12 May 2020.
- ^ "Latuda to Finally Hit Japan Market on June 11". PHARMA JAPAN. Archived from the original on 10 October 2022. Retrieved 10 October 2022.
- ^ "Sumitomo Dainippon Pharma Announces Approval of Atypical Antipsychotic Agent, LATUDA Tablets in Japan". IR News | Investor Relations. Sumitomo Pharma. Archived from the original on 10 October 2022. Retrieved 10 October 2022.
- ^ "Kusuri-no-Shiori(Drug Information Sheet) Latuda tablets". Archived from the original on 10 October 2022. Retrieved 10 October 2022.
- PMID 33679481.
- PMID 20122369.
- PMID 19903574.
- .
- PMID 18728771.
- PMID 14609883.
- PMID 23485111.
- PMID 16841630.
- PMID 22868059.
- S2CID 25512763.
- PMID 28590601.
- ^ "Lurasidone". MedlinePlus. U.S. National Library of Medicine. Archived from the original on 23 January 2019. Retrieved 11 September 2018.
- PMID 24825095.
- ^ Pregnancy category
- PMID 18378767.
- PMID 21459738.
- ^ Office of the Commissioner (14 July 2021). "Grapefruit Juice and Some Drugs Don't Mix". FDA.
- from the original on 15 November 2022. Retrieved 15 November 2022.
- PMID 31098889.
- ^ "Lurasidone Demonstrated Efficacy in Treating Patients With Schizophrenia in Pivotal Phase 3 Study" (Press release). Dainippon Sumitomo Pharma. 26 August 2009. Archived from the original on 14 May 2015. Retrieved 3 October 2016.
- S2CID 32085212.
- ^ a b "Latuda: Prescribing Information". Psychotherapeutic Drugs. Archived from the original on 28 June 2011. Retrieved 17 December 2010.
- ^ "Latuda". Drugs.com. Archived from the original on 5 April 2019. Retrieved 17 December 2010.
- PMID 15169702.
- ^ "Latuda Prescribing Information" (PDF). Sunovion Pharmaceuticals. Archived (PDF) from the original on 12 July 2018. Retrieved 25 March 2014.
- PMID 26196189.
- PMID 26918425.
- S2CID 49227958.
- S2CID 76666344.
- ISBN 978-0-85369-845-6.
Withdrawal of antipsychotic drugs after long-term therapy should always be gradual and closely monitored to avoid the risk of acute withdrawal syndromes or rapid relapse.
- ^ ISBN 978-0-19-852748-0. Archivedfrom the original on 10 January 2023. Retrieved 8 May 2020.
- S2CID 6267180.
- ISBN 978-88-470-2679-7. Archivedfrom the original on 10 January 2023. Retrieved 8 May 2020.
- ^ Roth BL, Driscol J. "PDSP Ki Database". Psychoactive Drug Screening Program (PDSP). University of North Carolina at Chapel Hill and the United States National Institute of Mental Health. Archived from the original on 28 August 2021. Retrieved 14 August 2017.
- ^ S2CID 12893717.
- S2CID 25838798.
- ^ "(3aR,4S,7R,7aS)-2-[[(1R,2R)-2-[[4-(1,2-Benzisothiazol-3-yl)-1-piperazinyl]methyl]cyclohexyl]methyl]hexahydro-4,7-methano-1H-isoindole-1,3(2H)-dione". PubChem. U.S. National Library of Medicine. Archived from the original on 2 December 2022. Retrieved 2 December 2022.
- S2CID 236926992.
- S2CID 25628197.
- PMID 26316760.
- PMID 25453735.
- ^ Lincoln J, Tripathi A (2011). "Lurasidone for Schizophrenia". Current Psychiatry. 10 (1): 67–70. Archived from the original on 3 October 2016. Retrieved 2 October 2016.
- PMID 17662268.
- S2CID 207485482.
- PMID 21935296.
- S2CID 195699303.
- S2CID 253737308.
- PMID 26577488.
- ^ PMID 22570547.
- ^ a b c "Lurasidone pharmacology review" (PDF). Center for Drug Evaluation and Research. 30 December 2009. Archived (PDF) from the original on 3 October 2016. Retrieved 2 October 2016.
- ^ "European Medicines Agency Assessment report Latuda International non-proprietary name: LURASIDONE Procedure No. EMEA/H/C/002713/0000" (PDF). Archived (PDF) from the original on 14 November 2022. Retrieved 14 November 2022.
- ^ "Leading Latuda through the crowd". 1 October 2011. Archived from the original on 28 August 2018. Retrieved 25 June 2017.
Latuda was developed at an R&D facility established in Fort Lee, NJ, about eight years ago by Sunovion's Japanese parent Dainippon Sumitomo Pharma Co. (DSP).
- ISBN 978-0-12-411524-8. Archivedfrom the original on 11 April 2023. Retrieved 4 September 2017 – via Google Books.
- ^ "EXCLI Journal" (PDF). 17 November 2016. Archived from the original (PDF) on 17 November 2016.
- ^ Canadian Agency for Drugs and Technologies in Health (2014). "Summary of Pharmacoeconomic Submission". Lurasidone Hydrochloride (Latuda): Management of Manifestations of Schizophrenia. Canadian Agency for Drugs and Technologies in Health. Archived from the original on 28 August 2021. Retrieved 30 April 2020 – via National Center for Biotechnology Information, U.S. National Library of Medicine.
- PMID 28579725.
- ^ "'Lurasidone' drug search". CIMS India. Archived from the original on 28 August 2021. Retrieved 21 April 2018.
- ^ "Generic Drugs (ndrugs.com)". Archived from the original on 1 May 2018. Retrieved 30 April 2018.
- ^ "Drug Approval Package: Latuda (lurasidone hydrochloride) Tablets NDA #200603". U.S. Food and Drug Administration (FDA). 24 December 1999. Archived from the original on 25 February 2021. Retrieved 12 May 2020.
- ^ "FDA approves Latuda to treat schizophrenia in adults" (Press release). U.S. Food and Drug Administration (FDA). 28 October 2010. Archived from the original on 30 October 2010. Retrieved 29 October 2010.
- ^ "Lurasidone, 18.5mg, 37mg, 74mg film-coated tablets (Latuda) SMC No. (994/14)" (PDF). scottishmedicines.org.uk. Scottish Medicines Consortium. 2014. Archived from the original (PDF) on 8 March 2016. Retrieved 7 March 2016.
- ^ "Summary Basis of Decision (SBD) for Latuda". hc-sc.gc.ca. Health Canada. 2012. Archived from the original on 27 June 2013. Retrieved 19 June 2013.
- ^ "European Marketing Authorization for Latuda". takeda.com. Archived from the original on 26 December 2017. Retrieved 25 November 2015.
- ^ "Lurasidone: FDA-Approved Drugs". U.S. Food and Drug Administration (FDA). Archived from the original on 1 October 2020. Retrieved 12 May 2020.
