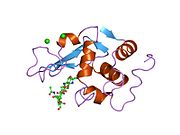
Lysozyme
| Lysozyme | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||
ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| Gene Ontology | AmiGO / QuickGO | ||||||||
| |||||||||
| Glycoside hydrolase, family 22, lysozyme | |
|---|---|
 Lysozyme crystals stained with methylene blue. | |
| Identifiers | |
| Symbol | ? |
| InterPro | IPR000974 |
Lysozyme (EC 3.2.1.17, muramidase, N-acetylmuramide glycanhydrolase; systematic name peptidoglycan N-acetylmuramoylhydrolase) is an antimicrobial enzyme produced by animals that forms part of the innate immune system. It is a glycoside hydrolase that catalyzes the following process:
- Hydrolysis of (1→4)-β-linkages between N-acetylmuramic acid and N-acetyl-D-glucosamine residues in a peptidoglycan and between N-acetyl-D-glucosamine residues in chitodextrins
Peptidoglycan is the major component of gram-positive bacterial cell wall.[1] This hydrolysis in turn compromises the integrity of bacterial cell walls causing lysis of the bacteria.
Lysozyme is abundant in
Hen egg white lysozyme is thermally stable, with a melting point reaching up to 72 °C at pH 5.0.[5] However, lysozyme in human milk loses activity very quickly at that temperature.[6] Hen egg white lysozyme maintains its activity in a large range of pH (6–9).[7] Its isoelectric point is 11.35.[8] The isoelectric point of human milk lysozyme is 10.5–11.[9]
Function and mechanism
The enzyme functions by hydrolyzing glycosidic bonds in peptidoglycans. The enzyme can also break glycosidic bonds in chitin, although not as effectively as true chitinases.[10]
Shorter saccharides like tetrasaccharide have also shown to be viable substrates but via an intermediate with a longer chain.[11] Chitin has also been shown to be a viable lysozyme substrate. Artificial substrates have also been developed and used in lysozyme.[12]
Mechanism
Phillips
The Phillips mechanism proposed that the enzyme's catalytic power came from both steric strain on the bound substrate and electrostatic stabilization of an oxo-carbenium intermediate. From X-ray crystallographic data, Phillips proposed the active site of the enzyme, where a hexasaccharide binds. The lysozyme distorts the fourth sugar (in the D or -1 subsite) in the hexasaccharide into a half-chair conformation. In this stressed state, the glycosidic bond is more easily broken.[13] An ionic intermediate containing an oxo-carbenium is created as a result of the glycosidic bond breaking.[14] Thus distortion causing the substrate molecule to adopt a strained conformation similar to that of the transition state will lower the energy barrier of the reaction.[15]
The proposed oxo-carbonium intermediate was speculated to be electrostatically stabilized by aspartate and glutamate residues in the active site by Arieh Warshel in 1978. The electrostatic stabilization argument was based on comparison to bulk water, the reorientation of water dipoles can cancel out the stabilizing energy of charge interaction. In Warshel's model, the enzyme acts as a super-solvent, which fixes the orientation of ion pairs and provides super-solvation (very good stabilization of ion pairs), and especially lower the energy when two ions are close to each other.[16]
The rate-determining step (RDS) in this mechanism is related to formation of the oxo-carbenium intermediate. There were some contradictory results to indicate the exact RDS. By tracing the formation of product (p-nitrophenol), it was discovered that the RDS can change over different temperatures, which was a reason for those contradictory results. At a higher temperature the RDS is formation of glycosyl enzyme intermediate and at a lower temperature the breakdown of that intermediate.[17]

Covalent mechanism

In an early debate in 1969, Dahlquist proposed a covalent mechanism for lysozyme based on kinetic isotope effect,[14] but for a long time the ionic mechanism was more accepted. In 2001, a revised mechanism was proposed by Vocadlo via a covalent but not ionic intermediate. Evidence from ESI-MS analysis indicated a covalent intermediate. A 2-fluoro substituted substrate was used to lower the reaction rate and accumulate an intermediate for characterization.[19] The amino acid side-chains glutamic acid 35 (Glu35) and aspartate 52 (Asp52) have been found to be critical to the activity of this enzyme. Glu35 acts as a proton donor to the glycosidic bond, cleaving the C-O bond in the substrate, whereas Asp52 acts as a nucleophile to generate a glycosyl enzyme intermediate. The Glu35 reacts with water to form hydroxyl ion, a stronger nucleophile than water, which then attacks the glycosyl enzyme intermediate, to give the product of hydrolysis and leaving the enzyme unchanged.[20] This type of covalent mechanism for enzyme catalysis was first proposed by Koshland.[21]
More recently, quantum mechanics/ molecular mechanics (QM/MM) molecular dynamics simulations have been using the crystal of HEWL and predict the existence of a covalent intermediate.[22] Evidence for the ESI-MS and X-ray structures indicate the existence of covalent intermediate, but primarily rely on using a less active mutant or non-native substrate. Thus, QM/MM molecular dynamics provides the unique ability to directly investigate the mechanism of wild-type HEWL and native substrate. The calculations revealed that the covalent intermediate from the covalent mechanism is ~30 kcal/mol more stable than the ionic intermediate from the Phillips mechanism.[22] These calculations demonstrate that the ionic intermediate is extremely energetically unfavorable and the covalent intermediates observed from experiments using less active mutant or non-native substrates provide useful insight into the mechanism of wild-type HEWL.

Inhibition
Imidazole derivatives can form a charge-transfer complex with some residues (in or outside active center) to achieve a competitive inhibition of lysozyme.[23] In Gram-negative bacteria, the lipopolysaccharide acts as a non-competitive inhibitor by highly favored binding with lysozyme.[24]
Non-enzymatic action
Despite that the muramidase activity of lysozyme has been supposed to play the key role for its antibacterial properties, evidence of its non-enzymatic action was also reported. For example, blocking the catalytic activity of lysozyme by mutation of critical amino acid in the active site (52-
Enzyme conformation changes
Lysozyme exhibits two conformations: an open active state and a closed inactive state. The catalytic relevance was examined with single walled carbon nanotubes (SWCN) field effect transistors (FETs), where a singular lysozyme was bound to the SWCN FET.[28] Electronically monitoring the lysozyme showed two conformations, an open active site and a closed inactive site. In its active state lysozyme is able to processively hydrolyze its substrate, breaking on average 100 bonds at a rate of 15 per second. In order to bind a new substrate and move from the closed inactive state to the open active state requires two conformation step changes, while inactivation requires one step.
Superfamily
The conventional C-type lysozyme is part of a larger group of structurally and mechanistically related enzymes termed the lysozyme superfamily. This family unites GH22 C-type ("chicken") lysozymes with plant chitinase GH19, G-type ("goose") lysozyme GH23, V-type ("viral") lysozyme GH24 and the chitosanase GH46 families. The lysozyme-type nomenclature only reflects the source a type is originally isolated from and does not fully reflect the taxonomic distribution.[29] For example, humans and many other mammals have two G-type lysozyme genes, LYG1 and LYG2.[30]
Role in disease and therapy
| LYZ | ||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||
| Wikidata | ||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||
Lysozyme is part of the innate immune system. Reduced lysozyme levels have been associated with
Since lysozyme is a natural form of protection from Gram-positive pathogens like Bacillus and Streptococcus,[38] it plays an important role in immunology of infants in human milk feeding.[39] Whereas the skin is a protective barrier due to its dryness and acidity, the conjunctiva (membrane covering the eye) is, instead, protected by secreted enzymes, mainly lysozyme and defensin. However, when these protective barriers fail, conjunctivitis results.
In certain cancers (especially myelomonocytic leukemia) excessive production of lysozyme by cancer cells can lead to toxic levels of lysozyme in the blood. High lysozyme blood levels can lead to kidney failure and low blood potassium, conditions that may improve or resolve with treatment of the primary malignancy.
Serum lysozyme is much less specific for diagnosis of sarcoidosis than serum angiotensin converting enzyme; however, since it is more sensitive, it is used as a marker of sarcoidosis disease activity and is suitable for disease monitoring in proven cases.[40]
Chemical synthesis
The first chemical synthesis of a lysozyme protein was attempted by Prof. George W. Kenner and his group at the University of Liverpool in England.[41] This was finally achieved in 2007 by Thomas Durek in Steve Kent's lab at the University of Chicago who made a synthetic functional lysozyme molecule.[42]
Other applications
Lysozyme crystals have been used to grow other functional materials for catalysis and biomedical applications.[43][44][45] Lysozyme is a commonly used enzyme for lysing gram positive bacteria.[46] Due to the unique function of lysozyme in which it can digest the cell wall and causes osmotic shock (burst the cell by suddenly changing solute concentration around the cell and thus the osmotic pressure), lysozyme is commonly used in lab setting to release proteins from bacterium periplasm while the inner membrane remains sealed as vesicles called the spheroplast.[47][48]
For example, E. coli can be lysed using lysozyme to free the contents of the periplasmic space. It is especially useful in lab setting for trying to collect the contents of the periplasm.[1] Lysozyme treatment is optimal at particular temperatures, pH ranges, and salt concentrations. Lysozyme activity increases with increasing temperatures, up to 60 degrees Celsius, with a pH range of 6.0-7.0. The salts present also affect lysozyme treatment, where some assert inhibitory effects, and others promote lysis via lysozyme treatment. Sodium chloride induces lysis, but at high concentrations, it is an active inhibitor of lysis. Similar observations have been seen with the use of potassium salts. Slight variations are present due to differences in bacterial strains.[49] A consequence of the use of lysozyme in extracting recombinant proteins for protein crystallization is that the crystal may be contaminated with units of lysozyme, producing a physiologically irrelevant combination. In fact, some proteins simply cannot crystalize without such contamination.[50][51]
Furthermore, lysozyme can serve as a tool in the expression of toxic recombinant proteins. Expressing recombinant proteins in BL21(DE3) strains is typically accomplished by the T7-RNA-polymerase. Via IPTG induction, the UV-5 repressor is inhibited, leading to the transcription of the T7-RNA-polymerase and thereby of the protein of interest. Nonetheless, a basal level of the T7-RNA-polymerase is observable even without induction. T7 lysozyme acts as an inhibitor of the T7-RNA-polymerase. Newly invented strains, containing a helper plasmid (pLysS), constitutively co-express low levels of T7 lysozyme, providing high stringency and consistent expression of the toxic recombinant protein.[52]
History
The antibacterial property of hen egg white, due to the lysozyme it contains, was first observed by Laschtschenko in 1909.[53] The bacteria-killing activity of nasal mucus was demonstrated in 1922 by Alexander Fleming, the discoverer of penicillin, who coined the term "lysozyme".[54] He is reported as saying: "As this substance has properties akin to those of ferments I have called it a 'Lysozyme'."[55] Fleming went on to show that an enzymic substance was present in a wide variety of secretions and was capable of rapidly lysing (i.e. dissolving) different bacteria, particularly a yellow "coccus" that he studied".[56]
Lysozyme was first crystallised by
See also
References
- ^ ISBN 978-0-8493-8935-1.
- ^ Williams S, Vocadlo D. "Glycoside hydrolase family 22". Cazypedia. Retrieved 11 April 2017.
- PMID 2829884.
- PMID 2546758.
- PMID 23833521.
- S2CID 4215401.
- ^ "Lysozyme, Product information" (PDF). Sigma-Aldrich.
- ^ "Lysozyme, Product information" (PDF). Sigma-Aldrich.
- PMID 5778672.
- S2CID 27906558.
- S2CID 31794497.
- )
- S2CID 35094695.
- ^ PMID 5388150.
- PMID 2069076.
- PMID 281676.
- PMID 6773958.
- ^ "Hen Egg-White (HEW) Lysozyme - Proteopedia, life in 3D".
- ^ S2CID 205020153.
- ISBN 978-0-495-11912-8.
- S2CID 86709302.
- ^ PMID 18802578.
- PMID 5063023.
- PMID 2647736.
- S2CID 21593262.
- PMID 29233221.
- ISBN 978-0-8493-9225-2.
- PMID 22267809.
- PMID 21085702.
- PMID 25167808.
- ^ a b c GRCh38: Ensembl release 89: ENSG00000090382 – Ensembl, May 2017
- ^ a b c GRCm38: Ensembl release 89: ENSMUSG00000069515 – Ensembl, May 2017
- ^ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- PMID 1640295.
- PMID 23516474.
- ^ Molteni M (30 June 2016). "Spilled Milk". Case Studies: News Features. Undark: Truth, Beauty, Science. Retrieved 12 January 2017.
- ISBN 978-0-07-110706-8.
- PMID 362248.
- S2CID 3999327.
- S2CID 170906912.
- PMID 17360367.
- PMID 21278750.
- PMID 23211931.
- PMID 23813903.
- PMID 13373865.
- ISBN 978-0-511-53532-1.
- ISBN 978-0-470-08766-4.
- PMID 13436356.
- PMID 22297987.
- PMID 35312352.
- PMID 11126126.
- S2CID 456259.
- S2CID 206011471.
- JSTOR 80959.
- ISBN 978-0-08-058214-6.
- S2CID 4161467.
- S2CID 10234792.
- S2CID 2629199.
- PMID 14063294.
- S2CID 12870128.
- S2CID 33906706.
- S2CID 31794497.
External links
- Muramidase at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
- Proteopedia.org HEW Lysozyme
- PDBe-KB provides an overview of all the structure information available in the PDB for Human Lysozyme C.
- PDBe-KB provides an overview of all the structure information available in the PDB for Hen egg white Lysozyme C.