Messenger RNA
In
.mRNA is created during the process of
As in
The concept of mRNA was developed by Sydney Brenner and Francis Crick in 1960 during a conversation with François Jacob. In 1961, mRNA was identified and described independently by one team consisting of Brenner, Jacob, and Matthew Meselson, and another team led by James Watson. While analyzing the data in preparation for publication, Jacob and Jacques Monod coined the name "messenger RNA".
Synthesis
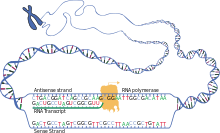
The brief existence of an mRNA molecule begins with transcription, and ultimately ends in degradation. During its life, an mRNA molecule may also be processed, edited, and transported prior to translation. Eukaryotic mRNA molecules often require extensive processing and transport, while
Transcription
Transcription is when RNA is copied from DNA. During transcription,
Eukaryotic pre-mRNA processing

Processing of mRNA differs greatly among eukaryotes, bacteria, and archaea. Non-eukaryotic mRNA is, in essence, mature upon transcription and requires no processing, except in rare cases.[1] Eukaryotic pre-mRNA, however, requires several processing steps before its transport to the cytoplasm and its translation by the ribosome.
Splicing
The extensive processing of eukaryotic pre-mRNA that leads to the mature mRNA is the RNA splicing, a mechanism by which introns or outrons (non-coding regions) are removed and exons (coding regions) are joined.[citation needed]
5' cap addition
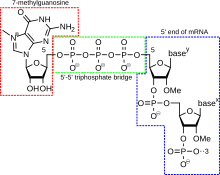
A 5' cap (also termed an RNA cap, an RNA
Cap addition is coupled to transcription, and occurs co-transcriptionally, such that each influences the other. Shortly after the start of transcription, the 5' end of the mRNA being synthesized is bound by a
Editing
In some instances, an mRNA will be edited, changing the nucleotide composition of that mRNA. An example in humans is the apolipoprotein B mRNA, which is edited in some tissues, but not others. The editing creates an early stop codon, which, upon translation, produces a shorter protein.
Polyadenylation

Polyadenylation is the covalent linkage of a polyadenylyl moiety to a messenger RNA molecule. In eukaryotic organisms most messenger RNA (mRNA) molecules are polyadenylated at the 3' end, but recent studies have shown that short stretches of uridine (oligouridylation) are also common.[2] The poly(A) tail and the protein bound to it aid in protecting mRNA from degradation by exonucleases. Polyadenylation is also important for transcription termination, export of the mRNA from the nucleus, and translation. mRNA can also be polyadenylated in prokaryotic organisms, where poly(A) tails act to facilitate, rather than impede, exonucleolytic degradation.[citation needed]
Polyadenylation occurs during and/or immediately after transcription of DNA into RNA. After transcription has been terminated, the mRNA chain is cleaved through the action of an endonuclease complex associated with RNA polymerase. After the mRNA has been cleaved, around 250 adenosine residues are added to the free 3' end at the cleavage site. This reaction is catalyzed by
Polyadenylation site mutations also occur. The primary RNA transcript of a gene is cleaved at the poly-A addition site, and 100–200 A's are added to the 3' end of the RNA. If this site is altered, an abnormally long and unstable mRNA construct will be formed.
Transport
Another difference between eukaryotes and prokaryotes is mRNA transport. Because eukaryotic transcription and translation is compartmentally separated, eukaryotic mRNAs must be exported from the nucleus to the cytoplasm—a process that may be regulated by different signaling pathways.[3] Mature mRNAs are recognized by their processed modifications and then exported through the nuclear pore by binding to the cap-binding proteins CBP20 and CBP80,[4] as well as the transcription/export complex (TREX).[5][6] Multiple mRNA export pathways have been identified in eukaryotes.[7]
In spatially complex cells, some mRNAs are transported to particular subcellular destinations. In mature
Translation
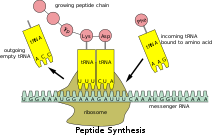
Because prokaryotic mRNA does not need to be processed or transported, translation by the ribosome can begin immediately after the end of transcription. Therefore, it can be said that prokaryotic translation is coupled to transcription and occurs co-transcriptionally.[citation needed]
Eukaryotic mRNA that has been processed and transported to the cytoplasm (i.e., mature mRNA) can then be translated by the ribosome. Translation may occur at ribosomes free-floating in the cytoplasm, or directed to the endoplasmic reticulum by the signal recognition particle. Therefore, unlike in prokaryotes, eukaryotic translation is not directly coupled to transcription. It is even possible in some contexts that reduced mRNA levels are accompanied by increased protein levels, as has been observed for mRNA/protein levels of EEF1A1 in breast cancer.[17][non-primary source needed]
Structure
Coding regions
Coding regions are composed of
Untranslated regions

Untranslated regions (UTRs) are sections of the mRNA before the start codon and after the stop codon that are not translated, termed the five prime untranslated region (5' UTR) and three prime untranslated region (3' UTR), respectively. These regions are transcribed with the coding region and thus are exonic as they are present in the mature mRNA. Several roles in gene expression have been attributed to the untranslated regions, including mRNA stability, mRNA localization, and translational efficiency. The ability of a UTR to perform these functions depends on the sequence of the UTR and can differ between mRNAs. Genetic variants in 3' UTR have also been implicated in disease susceptibility because of the change in RNA structure and protein translation.[20]
The stability of mRNAs may be controlled by the 5' UTR and/or 3' UTR due to varying affinity for RNA degrading enzymes called ribonucleases and for ancillary proteins that can promote or inhibit RNA degradation. (See also, C-rich stability element.)
Translational efficiency, including sometimes the complete inhibition of translation, can be controlled by UTRs. Proteins that bind to either the 3' or 5' UTR may affect translation by influencing the ribosome's ability to bind to the mRNA.
Cytoplasmic localization of mRNA is thought to be a function of the 3' UTR. Proteins that are needed in a particular region of the cell can also be translated there; in such a case, the 3' UTR may contain sequences that allow the transcript to be localized to this region for translation.
Some of the elements contained in untranslated regions form a characteristic
Poly(A) tail
The 3' poly(A) tail is a long sequence of
Monocistronic versus polycistronic mRNA
An mRNA molecule is said to be monocistronic when it contains the genetic information to
mRNA circularization

In eukaryotes mRNA molecules form circular structures due to an interaction between the eIF4E and poly(A)-binding protein, which both bind to eIF4G, forming an mRNA-protein-mRNA bridge.[24] Circularization is thought to promote cycling of ribosomes on the mRNA leading to time-efficient translation, and may also function to ensure only intact mRNA are translated (partially degraded mRNA characteristically have no m7G cap, or no poly-A tail).[25]
Other mechanisms for circularization exist, particularly in virus mRNA.
RNA virus genomes (the + strands of which are translated as mRNA) are also commonly circularized.[26] During genome replication the circularization acts to enhance genome replication speeds, cycling viral RNA-dependent RNA polymerase much the same as the ribosome is hypothesized to cycle.
Degradation
Different mRNAs within the same cell have distinct lifetimes (stabilities). In bacterial cells, individual mRNAs can survive from seconds to more than an hour. However, the lifetime averages between 1 and 3 minutes, making bacterial mRNA much less stable than eukaryotic mRNA.[27] In mammalian cells, mRNA lifetimes range from several minutes to days.[28] The greater the stability of an mRNA the more protein may be produced from that mRNA. The limited lifetime of mRNA enables a cell to alter protein synthesis rapidly in response to its changing needs. There are many mechanisms that lead to the destruction of an mRNA, some of which are described below.
Prokaryotic mRNA degradation
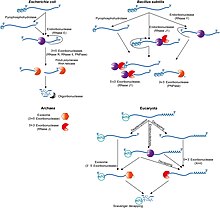
In general, in prokaryotes the lifetime of mRNA is much shorter than in eukaryotes. Prokaryotes degrade messages by using a combination of ribonucleases, including
Eukaryotic mRNA turnover
Inside eukaryotic cells, there is a balance between the processes of
AU-rich element decay
The presence of
Nonsense-mediated decay
Eukaryotic messages are subject to surveillance by
Small interfering RNA (siRNA)
In
MicroRNA (miRNA)
MicroRNAs (miRNAs) are small RNAs that typically are partially complementary to sequences in metazoan messenger RNAs.[37][38] Binding of a miRNA to a message can repress translation of that message and accelerate poly(A) tail removal, thereby hastening mRNA degradation. The mechanism of action of miRNAs is the subject of active research.[39][40]
Other decay mechanisms
There are other ways by which messages can be degraded, including non-stop decay and silencing by Piwi-interacting RNA (piRNA), among others.
Applications
The administration of a nucleoside-modified messenger RNA sequence can cause a cell to make a protein, which in turn could directly treat a disease or could function as a vaccine; more indirectly the protein could drive an endogenous stem cell to differentiate in a desired way.[41][42]
The primary challenges of RNA therapy center on delivering the RNA to the appropriate cells.
Overcoming these challenges, mRNA as a therapeutic was first put forward in 1989 "after the development of a broadly applicable in vitro transfection technique."[44] In the 1990s, mRNA vaccines for personalized cancer have been developed, relying on non-nucleoside modified mRNA. mRNA based therapies continue to be investigated as a method of treatment or therapy for both cancer as well as auto-immune, metabolic, and respiratory inflammatory diseases. Gene editing therapies such as CRISPR may also benefit from using mRNA to induce cells to make the desired Cas protein.[45]
Since the 2010s, RNA vaccines and other RNA therapeutics have been considered to be "a new class of drugs".[46] The first mRNA-based vaccines received restricted authorization and were rolled out across the world during the COVID-19 pandemic by Pfizer–BioNTech COVID-19 vaccine and Moderna, for example.[47] The 2023 Nobel Prize in Physiology or Medicine was awarded to Katalin Karikó and Drew Weissman for the development of effective mRNA vaccines against COVID-19.[48][49][50]
History
Several molecular biology studies during the 1950s indicated that RNA played some kind of role in protein synthesis, but that role was not clearly understood. For instance, in one of the earliest reports,
The idea of mRNA was first conceived by Sydney Brenner and Francis Crick on 15 April 1960 at King's College, Cambridge, while François Jacob was telling them about a recent experiment conducted by Arthur Pardee, himself, and Monod (the so-called PaJaMo experiment, which did not prove mRNA existed but suggested the possibility of its existence). With Crick's encouragement, Brenner and Jacob immediately set out to test this new hypothesis, and they contacted Matthew Meselson at the California Institute of Technology for assistance. During the summer of 1960, Brenner, Jacob, and Meselson conducted an experiment in Meselson's laboratory at Caltech which was the first to prove the existence of mRNA. That fall, Jacob and Monod coined the name "messenger RNA" and developed the first theoretical framework to explain its function.[54]
In February 1961, James Watson revealed that his Harvard-based research group had been right behind them with a series of experiments whose results pointed in roughly the same direction. Brenner and the others agreed to Watson's request to delay publication of their research findings. As a result, the Brenner and Watson articles were published simultaneously in the same issue of Nature in May 1961, while that same month, Jacob and Monod published their theoretical framework for mRNA in the Journal of Molecular Biology.[54]
See also
- Extension Poly(A) Test
- GeneCalling, an mRNA profiling technology
- Missense mRNA
- mRNA display
- mRNA surveillance
- Prokaryotic mRNA degradation
- Transcriptome, the sum of all RNA in a cell
References
- ISBN 9780321851499.
- PMID 22291204.
- PMID 23427269.
- PMID 19453442.
- S2CID 1112194.
- PMID 19229134.
- PMID 21533221.
- PMID 7062109.
- S2CID 13395819.
- S2CID 5275219.
- PMID 12573215.
- S2CID 2453397.
- PMID 9789325.
- PMID 9281585.
- PMID 29078295.
- S2CID 229689880.
- PMID 30224719.
- PMID 16682450.
- PMID 12952875.
- PMID 26531896.
- ^ a b
Kozak M (March 1983). "Comparison of initiation of protein synthesis in procaryotes, eucaryotes, and organelles". Microbiological Reviews. 47 (1): 1–45. PMID 6343825.
- S2CID 4349134.
- PMID 21854988.
- PMID 9702200.
- PMID 16238092.
- PMID 35784275.
- OCLC 456641931.
- PMID 11486027.
- S2CID 4321451.
- PMID 17349952.
- S2CID 14817671.
- PMID 16364915.
- S2CID 40332253.
- PMID 8578590.
- PMID 17671086.
- PMID 18926973.
- ^ Robert E. Farrell, Jr. RNA Methodologies, 5th Edition. Academic Press, 2017
- PMID 15723116.
- ^ Tasuku Honjo, Michael Reth, Andreas Radbruch, Frederick Alt. Molecular Biology of B Cells, 2nd Edition. Academic Press, 2014 (including "updated research on microRNAs")
- PMID 19029310.
- ^ .
- ^ a b Gousseinov E, Kozlov M, Scanlan C (September 15, 2015). "RNA-Based Therapeutics and Vaccines". Genetic Engineering News.
- PMID 28655327.
- PMID 23064118.
- ^ Haridi R (2021-04-23). "The mRNA revolution: How COVID-19 hit fast-forward on an experimental technology". New Atlas. Retrieved 2021-04-26.
- PMID 25150148
- S2CID 248667843.
- ^ "The Nobel Prize in Physiology or Medicine 2023". NobelPrize.org. Retrieved 2023-10-03.
- ^ "Hungarian and US scientists win Nobel for COVID-19 vaccine discoveries". Reuters. 2023-10-02. Retrieved 2023-10-03.
- ^ "The Nobel Prize in Physiology or Medicine 2023". NobelPrize.org. Retrieved 2023-10-03.
- PMID 13032175.
- PMID 16589470.
- PMID 13069681.
- ^ PMID 26126273.
Further reading
- Alsaweed M, Lai CT, Hartmann PE, Geddes DT, Kakulas F (February 2016). "Human milk miRNAs primarily originate from the mammary gland resulting in unique miRNA profiles of fractionated milk". Scientific Reports. 6 (1): 20680. PMID 26854194.
- PMID 22980048.
- Melnik BC, Kakulas F, Geddes DT, Hartmann PE, John SM, Carrera-Bastos P, Cordain L, Schmitz G (21 June 2016). "Milk miRNAs: simple nutrients or systemic functional regulators?". Nutrition & Metabolism. 13 (1): 42. PMID 27330539.
- Vickers MH (June 2014). "Early life nutrition, epigenetics and programming of later life disease". Nutrients. 6 (6): 2165–2178. PMID 24892374.
- Zhou Q, Li M, Wang X, Li Q, Wang T, Zhu Q, Zhou X, Wang X, Gao X, Li X (2012). "Immune-related microRNAs are abundant in breast milk exosomes". International Journal of Biological Sciences. 8 (1): 118–123. PMID 22211110.
- Krause W (2023). "mRNA — From COVID-19 Treatment to Cancer Immunotherapy". Biomedicines. 11 (2): 308. PMID 36830845.
External links
- RNAi Atlas: a database of RNAi libraries and their target analysis results
- miRSearch Archived 2012-12-04 at the Wayback Machine: Tool for finding microRNAs that target mRNA
- How mRNA is coded?: YouTube video
- What is mRNA?: theconversation.com
