Marine biogeochemical cycles
| Part of a series of overviews on |
| Marine life |
|---|
 |
Marine biogeochemical cycles are
There are biogeochemical cycles for the elements calcium, carbon, hydrogen, mercury, nitrogen, oxygen, phosphorus, selenium, and sulfur; molecular cycles for water and silica; macroscopic cycles such as the rock cycle; as well as human-induced cycles for synthetic compounds such as polychlorinated biphenyl (PCB). In some cycles there are reservoirs where a substance can be stored for a long time. The cycling of these elements is interconnected.
Overview
Energy flows directionally through ecosystems, entering as sunlight (or inorganic molecules for chemoautotrophs) and leaving as heat during the many transfers between trophic levels. However, the matter that makes up living organisms is conserved and recycled. The six most common elements associated with organic molecules—carbon, nitrogen, hydrogen, oxygen, phosphorus, and sulfur—take a variety of chemical forms and may exist for long periods in the atmosphere, on land, in water, or beneath the Earth's surface. Geologic processes, such as weathering, erosion, water drainage, and the subduction of the continental plates, all play a role in this recycling of materials. Because geology and chemistry have major roles in the study of this process, the recycling of inorganic matter between living organisms and their environment is called a biogeochemical cycle.[1]
The six aforementioned elements are used by organisms in a variety of ways. Hydrogen and oxygen are found in water and organic molecules, both of which are essential to life. Carbon is found in all organic molecules, whereas nitrogen is an important component of nucleic acids and proteins. Phosphorus is used to make nucleic acids and the phospholipids that comprise biological membranes. Sulfur is critical to the three-dimensional shape of proteins. The cycling of these elements is interconnected. For example, the movement of water is critical for leaching sulfur and phosphorus into rivers which can then flow into oceans. Minerals cycle through the biosphere between the biotic and abiotic components and from one organism to another.[2]
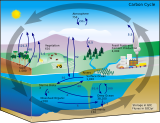
The water cycle
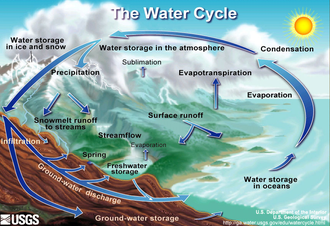
Water is the medium of the oceans, the medium which carries all the substances and elements involved in the marine biogeochemical cycles. Water as found in nature almost always includes dissolved substances, so water has been described as the "universal solvent" for its ability to dissolve so many substances.[3][4] This ability allows it to be the "solvent of life"[5] Water is also the only common substance that exists as solid, liquid, and gas in normal terrestrial conditions.[6] Since liquid water flows, ocean waters cycle and flow in currents around the world. Since water easily changes phase, it can be carried into the atmosphere as water vapour or frozen as an iceberg. It can then precipitate or melt to become liquid water again. All marine life is immersed in water, the matrix and womb of life itself.[7] Water can be broken down into its constituent hydrogen and oxygen by metabolic or abiotic processes, and later recombined to become water again.
While the water cycle is itself a
Ocean salinity
-
Annual mean sea surface salinity, measured in 2009 inpractical salinity units [14]
-
Vertical differences in sea salinity between the surface and a depth of 300 metres. Salinity increases with depth in red regions and decreases in blue regions.[15]
Sea spray
A stream of airborne microorganisms circles the planet above weather systems but below commercial air lanes.[16] Some peripatetic microorganisms are swept up from terrestrial dust storms, but most originate from marine microorganisms in sea spray. In 2018, scientists reported that hundreds of millions of viruses and tens of millions of bacteria are deposited daily on every square meter around the planet.[17][18] This is another example of water facilitating the transport of organic material over great distances, in this case in the form of live microorganisms.
Dissolved salt does not evaporate back into the atmosphere like water, but it does form
Ocean circulation

Solar radiation affects the oceans: warm water from the Equator tends to circulate toward the
As it travels poleward, warm water transported by strong warm water current undergoes evaporative cooling. The cooling is wind driven: wind moving over water cools the water and also causes
Winds drive ocean currents in the upper 100 meters of the ocean's surface. However, ocean currents also flow thousands of meters below the surface. These deep-ocean currents are driven by differences in the water's density, which is controlled by temperature (thermo) and salinity (haline). This process is known as thermohaline circulation. In the Earth's polar regions ocean water gets very cold, forming sea ice. As a consequence the surrounding seawater gets saltier, because when sea ice forms, the salt is left behind. As the seawater gets saltier, its density increases, and it starts to sink. Surface water is pulled in to replace the sinking water, which in turn eventually becomes cold and salty enough to sink. This initiates the deep-ocean currents driving the global conveyor belt.[30]
Thermohaline circulation drives a global-scale system of currents called the “global conveyor belt.” The conveyor belt begins on the surface of the ocean near the pole in the North Atlantic. Here, the water is chilled by Arctic temperatures. It also gets saltier because when sea ice forms, the salt does not freeze and is left behind in the surrounding water. The cold water is now more dense, due to the added salts, and sinks toward the ocean bottom. Surface water moves in to replace the sinking water, thus creating a current. This deep water moves south, between the continents, past the equator, and down to the ends of Africa and South America. The current travels around the edge of Antarctica, where the water cools and sinks again, as it does in the North Atlantic. Thus, the conveyor belt gets "recharged." As it moves around Antarctica, two sections split off the conveyor and turn northward. One section moves into the Indian Ocean, the other into the Pacific Ocean. These two sections that split off warm up and become less dense as they travel northward toward the equator, so that they rise to the surface (upwelling). They then loop back southward and westward to the South Atlantic, eventually returning to the North Atlantic, where the cycle begins again. The conveyor belt moves at much slower speeds (a few centimeters per second) than wind-driven or tidal currents (tens to hundreds of centimeters per second). It is estimated that any given cubic meter of water takes about 1,000 years to complete the journey along the global conveyor belt. In addition, the conveyor moves an immense volume of water—more than 100 times the flow of the Amazon River (Ross, 1995). The conveyor belt is also a vital component of the global ocean nutrient and carbon dioxide cycles. Warm surface waters are depleted of nutrients and carbon dioxide, but they are enriched again as they travel through the conveyor belt as deep or bottom layers. The base of the world's food chain depends on the cool, nutrient-rich waters that support the growth of algae and seaweed.[31]
| Average reservoir residence times [32] | |
|---|---|
| Reservoir | Average residence time |
| Antarctica | 20,000 years |
| Oceans | 3,200 years |
| Glaciers | 20 to 100 years |
| Seasonal snow cover | 2 to 6 months |
| Soil moisture | 1 to 2 months |
| Groundwater: shallow | 100 to 200 years |
| Groundwater: deep | 10,000 years |
| Lakes (see lake retention time) | 50 to 100 years |
| Rivers | 2 to 6 months |
| Atmosphere | 9 days |
The global average residence time of a water molecule in the ocean is about 3,200 years. By comparison the average residence time in the atmosphere is about nine days. If it is frozen in the Antarctic or drawn into deep groundwater it can be sequestered for ten thousand years.[32][33]
Cycling of key elements
| Some key elements involved in marine biogeochemical cycles | ||
|---|---|---|
Element
|
Diagram | Description |
Carbon
|

|
The acid-base chemistry in the oceans.
|
Oxygen
|

|
The molecular oxygen (O2) since it is the common product or reactant of many biogeochemical redox reactions within the cycle.[37] Processes within the oxygen cycle are considered to be biological or geological and are evaluated as either a source (O2 production) or sink (O2 consumption).[36][37]
|
Hydrogen
|

|
The hydrogen cycle consists of hydrogen exchanges between biotic (living) and abiotic (non-living) sources and sinks of hydrogen-containing compounds. Hydrogen (H) is the most abundant element in the universe.[38] On Earth, common H-containing inorganic molecules include water (H2O), hydrogen gas (H2), methane (CH4), hydrogen sulfide (H2S), and ammonia (NH3). Many organic compounds also contain H atoms, such as hydrocarbons and organic matter. Given the ubiquity of hydrogen atoms in inorganic and organic chemical compounds, the hydrogen cycle is focused on molecular hydrogen (H2). |
Nitrogen
|

|
The ecologists because nitrogen availability can affect the rate of key ecosystem processes, including primary production and decomposition. Human activities such as fossil fuel combustion, use of artificial nitrogen fertilizers, and release of nitrogen in wastewater have dramatically altered the global nitrogen cycle.[40][41][42] Human modification of the global nitrogen cycle can negatively affect the natural environment system and also human health.[43][44]
|
Phosphorus
|

|
The geologic time.[45] Humans have caused major changes to the global phosphorus cycle through shipping of phosphorus minerals, and use of phosphorus fertilizer , and also the shipping of food from farms to cities, where it is lost as effluent.
|
Sulphur
|

|
The essential element, being a constituent of many proteins and cofactors, and sulfur compounds can be used as oxidants or reductants in microbial respiration.[46] The global sulfur cycle involves the transformations of sulfur species through different oxidation states, which play an important role in both geological and biological processes. Earth's main sulfur sink is the oceans SO42−, where it is the major oxidizing agent.[47]
|
Iron
|

|
The microorganisms, especially iron-oxidizing bacteria. The abiotic processes include the rusting of iron-bearing metals, where Fe2+ is abiotically oxidized to Fe3+ in the presence of oxygen, and the reduction of Fe3+ to Fe2+ by iron-sulfide minerals. The biological cycling of Fe2+ is done by iron oxidizing and reducing microbes.[52][53]
|
Calcium
|

|
The calcium cycle is a transfer of calcium between dissolved and solid phases. There is a continuous supply of calcium ions into waterways from rocks, organisms, and soils.[54][55] Calcium ions are consumed and removed from aqueous environments as they react to form insoluble structures such as calcium carbonate and calcium silicate,[54][56] which can deposit to form sediments or the exoskeletons of organisms.[57] Calcium ions can also be utilized biologically, as calcium is essential to biological functions such as the production of bones and teeth or cellular function.[58][59] The calcium cycle is a common thread between terrestrial, marine, geological, and biological processes.[60] The marine calcium cycle is affected by changing atmospheric carbon dioxide due to ocean acidification.[57] |
Silicon
|

|
The silica between the Earth's systems. Opal silica (SiO2), also called silicon dioxide, is a chemical compound of silicon. Silicon is a bioessential element and is one of the most abundant elements on Earth.[61][62] The silica cycle has significant overlap with the carbon cycle (see the carbonate–silicate cycle) and plays an important role in the sequestration of carbon through continental weathering, biogenic export and burial as oozes on geologic timescales.[63]
|
Box models
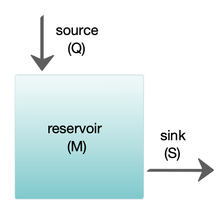
Box models are widely used to model biogeochemical systems.[65] Box models are simplified versions of complex systems, reducing them to boxes (or storage reservoirs) for chemical materials, linked by material fluxes (flows). Simple box models have a small number of boxes with properties, such as volume, that do not change with time. The boxes are assumed to behave as if they were mixed homogeneously.[64] These models are often used to derive analytical formulas describing the dynamics and steady-state abundance of the chemical species involved.
The diagram at the right shows a basic one-box model. The reservoir contains the amount of material M under consideration, as defined by chemical, physical or biological properties. The source Q is the flux of material into the reservoir, and the sink S is the flux of material out of the reservoir. The budget is the check and balance of the sources and sinks affecting material turnover in a reservoir. The reservoir is in a steady state if Q = S, that is, if the sources balance the sinks and there is no change over time.[64]
Global biogeochemical box models usually measure:
— reservoir masses in petagrams (Pg)
— flow fluxes in petagrams per year (Pg yr−1)
Diagrams in this article mostly use these units
________________________________________________
one
The
When two or more reservoirs are connected, the material can be regarded as cycling between the reservoirs, and there can be predictable patterns to the cyclic flow.[64] More complex multibox models are usually solved using numerical techniques.


example of a more complex model with many interacting boxes
The diagram above shows a simplified budget of ocean carbon flows. It is composed of three simple interconnected box models, one for the
Dissolved and particulate matter


Biological pumps
and carbon masses (Gt C) in dark boxes
The
The biological pump can be divided into three distinct phases,
Once this carbon is fixed into soft or hard tissue, the organisms either stay in the euphotic zone to be recycled as part of the regenerative nutrient cycle or once they die, continue to the second phase of the biological pump and begin to sink to the ocean floor. The sinking particles will often form aggregates as they sink, greatly increasing the sinking rate. It is this aggregation that gives particles a better chance of escaping predation and decomposition in the water column and eventually make it to the sea floor.
The fixed carbon that is either decomposed by bacteria on the way down or once on the sea floor then enters the final phase of the pump and is remineralized to be used again in primary production. The particles that escape these processes entirely are sequestered in the sediment and may remain there for millions of years. It is this sequestered carbon that is responsible for ultimately lowering atmospheric CO2.
| External videos | |
|---|---|
- Brum JR, Morris JJ, Décima M and Stukel MR (2014) "Mortality in the oceans: Causes and consequences". Eco-DAS IX Symposium Proceedings, Chapter 2, pages 16–48. Association for the Sciences of Limnology and Oceanography. ISBN 978-0-9845591-3-8.
- Mateus, M.D. (2017) "Bridging the gap between knowing and modeling viruses in marine systems—An upcoming frontier". Frontiers in Marine Science, 3: 284.
- Beckett, S.J. and Weitz, J.S. (2017) "Disentangling niche competition from grazing mortality in phytoplankton dilution experiments". PLOS ONE, 12(5): e0177517. .
Role of microorganisms
Carbon, oxygen and hydrogen cycles
The
Three main processes (or pumps) that make up the marine carbon cycle bring atmospheric
| Forms of carbon [80] | |||
|---|---|---|---|
| Carbon form | Chemical formula | State | Main reservoir |
| carbon dioxide | CO2 | gas | atmosphere |
| carbonic acid | H2CO3 | liquid | ocean |
bicarbonate ion
|
HCO3− | liquid (dissolved ion) |
ocean |
| organic compounds | Examples: C6H12O6 (glucose) CH4 (methane) |
solid gas |
organic sediments )
(fossil fuels |
| other carbon compounds | Examples: CaCO3 (calcium carbonate) CaMg(CO3)2 (calcium magnesium carbonate) |
solid | shells sedimentary rock |
Nitrogen and phosphorus cycles
The nitrogen cycle is as important in the ocean as it is on land. While the overall cycle is similar in both cases, there are different players and modes of transfer for nitrogen in the ocean.[81] Nitrogen enters the ocean through precipitation, runoff, or as N2 from the atmosphere. Nitrogen cannot be utilized by phytoplankton as N2 so it must undergo nitrogen fixation which is performed predominantly by cyanobacteria.[82] Without supplies of fixed nitrogen entering the marine cycle, the fixed nitrogen would be used up in about 2000 years.[83] Phytoplankton need nitrogen in biologically available forms for the initial synthesis of organic matter. Ammonia and urea are released into the water by excretion from plankton. Nitrogen sources are removed from the euphotic zone by the downward movement of the organic matter. This can occur from sinking of phytoplankton, vertical mixing, or sinking of waste of vertical migrators. The sinking results in ammonia being introduced at lower depths below the euphotic zone. Bacteria are able to convert ammonia to nitrite and nitrate but they are inhibited by light so this must occur below the euphotic zone.[82] Ammonification or mineralization is performed by bacteria to convert organic nitrogen to ammonia. Nitrification can then occur to convert the ammonium to nitrite and nitrate.[84] Nitrate can be returned to the euphotic zone by vertical mixing and upwelling where it can be taken up by phytoplankton to continue the cycle. N2 can be returned to the atmosphere through denitrification.
Ammonium is thought to be the preferred source of fixed nitrogen for phytoplankton because its assimilation does not involve a redox reaction and therefore requires little energy. Nitrate requires a redox reaction for assimilation but is more abundant so most phytoplankton have adapted to have the enzymes necessary to undertake this reduction (nitrate reductase). There are a few notable and well-known exceptions that include most Prochlorococcus and some Synechococcus that can only take up nitrogen as ammonium.[83]
Phosphorus is an essential nutrient for plants and animals. Phosphorus is a
Phosphorus occurs most abundantly in nature as part of the
Nutrient cycle

A
There is considerable overlap between the terms for the biogeochemical cycle and nutrient cycle. Some textbooks integrate the two and seem to treat them as synonymous terms.[88] However, the terms often appear independently. Nutrient cycle is more often used in direct reference to the idea of an intra-system cycle, where an ecosystem functions as a unit. From a practical point, it does not make sense to assess a terrestrial ecosystem by considering the full column of air above it as well as the great depths of Earth below it. While an ecosystem often has no clear boundary, as a working model it is practical to consider the functional community where the bulk of matter and energy transfer occurs.[89] Nutrient cycling occurs in ecosystems that participate in the "larger biogeochemical cycles of the earth through a system of inputs and outputs."[89]: 425
Dissolved nutrients
Nutrients dissolved in seawater are essential for the survival of marine life. Nitrogen and phosphorus are particularly important. They are regarded as
The process of cycling nutrients in the sea starts with biological pumping, when nutrients are extracted from surface waters by phytoplankton to become part of their organic makeup. Phytoplankton are either eaten by other organisms, or eventually die and drift down as marine snow. There they decay and return to the dissolved state, but at greater ocean depths. The fertility of the oceans depends on the abundance of the nutrients, and is measured by the primary production, which is the rate of fixation of carbon per unit of water per unit time. "Primary production is often mapped by satellites using the distribution of chlorophyll, which is a pigment produced by plants that absorbs energy during photosynthesis. The distribution of chlorophyll is shown in the figure above. You can see the highest abundance close to the coastlines where nutrients from the land are fed in by rivers. The other location where chlorophyll levels are high is in upwelling zones where nutrients are brought to the surface ocean from depth by the upwelling process..."[90]
"Another critical element for the health of the oceans is the dissolved oxygen content. Oxygen in the surface ocean is continuously added across the air-sea interface as well as by photosynthesis; it is used up in respiration by marine organisms and during the decay or oxidation of organic material that rains down in the ocean and is deposited on the ocean bottom. Most organisms require oxygen, thus its depletion has adverse effects for marine populations. Temperature also affects oxygen levels as warm waters can hold less dissolved oxygen than cold waters. This relationship will have major implications for future oceans, as we will see... The final seawater property we will consider is the content of dissolved CO2. CO2 is nearly opposite to oxygen in many chemical and biological processes; it is used up by plankton during photosynthesis and replenished during respiration as well as during the oxidation of organic matter. As we will see later, CO2 content has importance for the study of deep-water aging."[90]

Marine sulfur cycle


Sulfate reduction in the seabed is strongly focused toward near-surface sediments with high depositional rates along the ocean margins. The benthic marine sulfur cycle is therefore sensitive to anthropogenic influence, such as ocean warming and increased nutrient loading of coastal seas. This stimulates photosynthetic productivity and results in enhanced export of organic matter to the seafloor, often combined with low oxygen concentration in the bottom water (Rabalais et al., 2014; Breitburg et al., 2018). The biogeochemical zonation is thereby compressed toward the sediment surface, and the balance of organic matter mineralization is shifted from oxic and suboxic processes toward sulfate reduction and methanogenesis (Middelburg and Levin, 2009).[91]
The sulfur cycle in marine environments has been well-studied via the tool of sulfur isotope systematics expressed as δ34S. The modern global oceans have sulfur storage of 1.3 × 1021 g,[92] mainly occurring as sulfate with the δ34S value of +21‰.[93] The overall input flux is 1.0 × 1014 g/year with the sulfur isotope composition of ~3‰.[93] Riverine sulfate derived from the terrestrial weathering of sulfide minerals (δ34S = +6‰) is the primary input of sulfur to the oceans. Other sources are metamorphic and volcanic degassing and hydrothermal activity (δ34S = 0‰), which release reduced sulfur species (e.g., H2S and S0). There are two major outputs of sulfur from the oceans. The first sink is the burial of sulfate either as marine evaporites (e.g., gypsum) or carbonate-associated sulfate (CAS), which accounts for 6 × 1013 g/year (δ34S = +21‰). The second sulfur sink is pyrite burial in shelf sediments or deep seafloor sediments (4 × 1013 g/year; δ34S = -20‰).[94] The total marine sulfur output flux is 1.0 × 1014 g/year which matches the input fluxes, implying the modern marine sulfur budget is at steady state.[93] The residence time of sulfur in modern global oceans is 13,000,000 years.[95]
In modern oceans, Hydrogenovibrio crunogenus, Halothiobacillus, and Beggiatoa are primary sulfur oxidizing bacteria,[96][97] and form chemosynthetic symbioses with animal hosts.[98] The host provides metabolic substrates (e.g., CO2, O2, H2O) to the symbiont while the symbiont generates organic carbon for sustaining the metabolic activities of the host. The produced sulfate usually combines with the leached calcium ions to form gypsum, which can form widespread deposits on near mid-ocean spreading centers.[99]
Hydrothermal vents emit hydrogen sulfide that support the carbon fixation of chemolithotrophic bacteria that oxidize hydrogen sulfide with oxygen to produce elemental sulfur or sulfate.[96]
Iron cycle and dust

The
Iron in the ocean cycles between plankton, aggregated particulates (non-bioavailable iron), and dissolved (bioavailable iron), and becomes sediments through burial.[100][105][106] Hydrothermal vents release ferrous iron to the ocean[107] in addition to oceanic iron inputs from land sources. Iron reaches the atmosphere through volcanism,[108] aeolian wind,[109] and some via combustion by humans. In the Anthropocene, iron is removed from mines in the crust and a portion re-deposited in waste repositories.[103][106]

interact with other bacteria to acquire iron from dust
b. Trichodesmium can establish massive blooms in nutrient poor ocean regions with high dust deposition, partly due to their unique ability to capture dust, center it, and subsequently dissolve it.
c. Proposed dust-bound Fe acquisition pathway: Bacteria residing within the colonies produce siderophores (C-I) that react with the dust particles in the colony core and generate dissolved Fe (C-II). This dissolved Fe, complexed by siderophores, is then acquired by both Trichodesmium and its resident bacteria (C-III), resulting in a mutual benefit to both partners of the consortium.[110]
Iron is an essential micronutrient for almost every life form. It is a key component of hemoglobin, important to nitrogen fixation as part of the Nitrogenase enzyme family, and as part of the iron-sulfur core of ferredoxin it facilitates electron transport in chloroplasts, eukaryotic mitochondria, and bacteria. Due to the high reactivity of Fe2+ with oxygen and low solubility of Fe3+, iron is a limiting nutrient in most regions of the world.


![Annual mean sea surface salinity, measured in 2009 in practical salinity units [14]](http://upload.wikimedia.org/wikipedia/commons/thumb/6/61/SeaSurfaceSalinity.jpg/624px-SeaSurfaceSalinity.jpg)
![Vertical differences in sea salinity between the surface and a depth of 300 metres. Salinity increases with depth in red regions and decreases in blue regions.[15]](http://upload.wikimedia.org/wikipedia/commons/thumb/7/7f/Vertical_differences_in_ocean_salinity_%280_to_300m%29.png/540px-Vertical_differences_in_ocean_salinity_%280_to_300m%29.png)