Mecamylamine
 | |
| Clinical data | |
|---|---|
| Trade names | Inversine, Vecamyl |
| AHFS/Drugs.com | Consumer Drug Information |
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Protein binding | 40% |
| Identifiers | |
| |
JSmol) | |
| |
| |
| | |
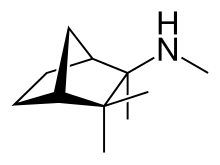
Mecamylamine (
Chemically, mecamylamine is a secondary
Medical uses
Mecamylamine has been used as an orally-active ganglionic blocker in treating autonomic dysreflexia and hypertension,[6] but, like most ganglionic blockers, it is more often used now as a research tool.
Mecamylamine is also sometimes used as an
In a recent double-blind, placebo-controlled Phase II trial in Indian patients with major depression, (S)-mecamylamine (TC-5214) appeared to have efficacy as an
Overdose
The LD50 for the HCl salt[13] in mice: 21 mg/kg (i.v.); 37 mg/kg (i.p.); 96 mg/kg (p.o.).[14]
Pharmacology
(S)-(+)-Mecamylamine
A large
A comprehensive review of the pharmacology of mecamylamine was published in 2001.[18]
History
Mecamylamine was brought to market by Merck & Co. in the 1950s; in 1996 Merck sold the asset to Layton Bioscience.[19] In 2002, Targacept acquired it from Layton, intending to repurpose it for CNS conditions.[20]
Targacept voluntarily withdrew mecamylamine from the market in 2009
See also
- Bupropion
- Scopolamine
References
- ^ "Mecamylamine". drugs.com. Retrieved May 15, 2015.
- S2CID 25690407.
- ^ "Drug Profile: Mecamylamine - Targacept". AdisInsight. Springer Nature Switzerland AG.
- ^ "Drugs@FDA: FDA Approved Drug Products". www.accessdata.fda.gov. Retrieved 2017-10-16.
- PMID 13476377.
- ^ Soine TO (1966). Wilson CO, Gisvold O, Doerge RF (eds.). Textbook of Organic Medicinal and Pharmaceutical Chemistry (5th ed.). Philadelphia: Lippincott. pp. 468–546.
- PMID 12080428.
- PMID 19040552.
- S2CID 6395092.
- ^ Carroll J (8 November 2011). "Key AZ/Targacept depression drug flunks first Phase III test". Fiercebiotech.com. Retrieved 2011-11-09.
- ^ Hirschler B (8 November 2011). "AstraZeneca, Targacept drug fails depression test". Reuters.
- ^ "AstraZeneca Pipeline as of the 27th of January 2011". Retrieved 2011-11-09.
- ^ In view of the time period when these data were generated, they presumably refer to the HCl salt of the racemic drug
- PMID 13618559.
- PMID 11303054.
- PMID 14061006.
- PMID 2002445.
- PMID 11354389.
- PMID 12080428.
- ^ "Press release: Targacept, Inc. Acquires Marketed Drug To Expand Its CNS Portfolio | Evaluate". Targacept via Evaluate. August 27, 2002.
- ^ "Notification letter from Targacept" (PDF). FDA. June 4, 2009.
- ^ "Determination That INVERSINE (Mecamylamine Hydrochloride) Tablet and Six Other Drug Products Were Not Withdrawn From Sale for Reasons of Safety or Effectiveness". Federal Register. 28 July 2011.
- ^ "Press release: Manchester Announces FDA Approval of Vecamyl". Manchester Pharamceuticals via Evaluate. May 1, 2013.
- ^ Fidler B (13 February 2014). "Retrophin Shares Boom Following Manchester Pharma Buyout". Xconomy.
- ^ Fidler B (10 August 2015). "Shkreli Leads $90M Round for New Startup, Turing Pharma | Xconomy". Xconomy.
