Anoxic depolarization in the brain
This article may be too technical for most readers to understand. (November 2013) |
Anoxic depolarization is a progressive and uncontrollable
Neural signal under normal oxygen uptake

Neurons function in the
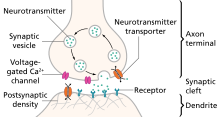
A chemical signal (
Brain energy crisis
Stroke onset
Within a few seconds of stroke onset, the brain responds by entering a state of metabolic depression, in which energy consumption is reduced to compensate for the reduction in energy production. Metabolic depression occurs as a result of suppressed synaptic transmission and hyperpolarization.
The suppression of synaptic transmission occurs because the presynaptic impulse temporarily fails to trigger the release of neurotransmitters, which, coupled with the altered ion conductance and a change in postsynaptic neuroreceptors, makes synapses unresponsive to neurotransmitter binding, thereby inhibiting postsynaptic excitation.[5]
Hyperpolarization, on the other hand, is employed to reduce neuronal activity by establishing a high threshold potential for firing across an action potential. This energy-conserving response is due to the continuous inward current of K+ ions, which help maintain the membrane
Imbalance in ion-homeostasis
Maintaining a balance between the intracellular and extracellular ionic concentrations at the postsynaptic terminal is critical to normal neuronal function. During oxygen depletion to the
The key player in the dramatic process of cationic influx is glutamate, an
During brain ischemia, glutamate is released in excess from the presynaptic terminal, leading to the uncontrollable opening of the
Post-anoxic depolarization: downstream neuronal damage

In the aftermath of anoxic depolarization, at the region of
Another
Selective vulnerability
Neurons are more susceptible to brain ischemia than the supporting
Selective vulnerability is how some parts of the brain are more sensitive to
While basal ganglia, cerebellar purkinje cells, hippocampal, and neocortical cells are more vulnerable to transient ischemic attack (TIA), brainstem and thalamic reticular neurons are more vulnerable to prolonged ischemic attack (stroke proper).[11] Meanwhile, the hippocampal pyramidal cells have been identified as the most vulnerable cells to ischemia.[12] One possible explanation for why selective vulnerability exists attributes the phenomenon to the different amounts of glutamate produced by different neurons, since it is glutamate release to the synaptic cleft that triggers Ca2+ influx, which in turn triggers biochemical processes that damage the neurons.[11] In other research, variation in the expression of immediate early gene and heat shock protein was identified as causing selective vulnerability.[12]
Anoxic-tolerance mechanisms
Metabolic depression
The painted turtle (Chrysemys picta) uses the mechanism of metabolic depression to combat oxygen depletion.[13] Within a few minutes of anoxia onset in the turtle's brain there is decreased cerebral blood flow that eventually ceases. Meanwhile, glycolysis is stimulated to maintain a near optimum ATP production.[3] This compensatory stimulation of glycolysis occurs because, in the turtle's brain, cytochrome a and a3 have a low affinity for oxygen.[13] Anaerobic glycolysis leads to lactate overload, which the turtle buffers to some extent by increased shell and bone CaCO3 production.[3]
However, glycolysis is not efficient for ATP production, and in order to maintain an optimum ATP concentration, the turtle's brain reduces its ATP consumption by suppressing its neuronal activity and gradually releasing
Pasteur effect
Another
Since the crucian carp has a more efficient strategy to prevent lactate buildup than C. picta, the initial glycolysis continues without ceasing, a process called the Pasteur effect.[14] In order to keep up with this fast glucose metabolism via glycolysis, as well as maintain the balance between ATP production and consumption, the crucian carp moderately suppresses its motor activities, releases GABA, and selectively suppresses some unnecessary sensory functions.[14] Crucian carp also counteracts the damaging effects of anoxia by swimming into cooler water, a phenomenon known as voluntary hypothermia.[3]
Tolerance in mammalian neonates
The brains of several mammalian neonates have been identified as able to confer resistance to anoxia in a fashion similar to that of the anoxic-tolerant aquatic organisms.
Research: neuroprotective agents

Currently, there is no effective way to combat stroke. The only FDA-
Many
See also
References
- ^ PMID 22864302.
- PMID 9428302.
- ^ PMID 15129179.
- ^ ISBN 9780878936977.
- ^ a b c d e f Lutz, P. L.; Nilsson, G. E. (1997). Neuroscience intelligence unit: The Brain Without Oxygen (2nd ed.). Austin, TX: Landes Bioscience and Chapman & Hall. pp. 1–207.
- PMID 23732543.
- PMID 23535140.
- PMID 20940167.
- ^ PMID 17684517.
- ^ PMID 18349449.
- ^ a b c Agamanolis, D. "Chapter 2: Cerebral Ischemia and Stroke". Neuropathology. Retrieved 4 November 2013.
- ^ PMID 20130351.
- ^ PMID 1348613.
- ^ ISBN 978-1-4419-9694-7.
- PMID 10487295.
- PMID 23065135.
External links
- The Ischemic Penumbra Archived 2020-09-20 at the Wayback Machine
- Lai TW, Shyu WC, Wang YT (2011). "Stroke intervention pathways: NMDA receptors and beyond". Trends Mol Med. 17 (5): 266–75. PMID 21310659.
