Microsporidia
| Microsporidia | |
|---|---|

| |
| Sporoblast of Fibrillanosema crangonycis | |
| Scientific classification | |
| Domain: | Eukaryota |
| Clade: | Amorphea |
| Clade: | Obazoa |
| (unranked): | Opisthokonta |
| Clade: | Holomycota |
| Kingdom: | Fungi |
| Subkingdom: | Rozellomyceta
|
| Class: | Microsporidiomycota Benny 2007 |
| Classes & orders[1] | |
| |
| Synonyms | |
Microsporidia are a group of
After infection they influence their hosts in various ways and all organs and tissues are invaded, though generally by different species of specialised microsporidia. Some species are lethal, and a few are used in biological control of insect pests. Parasitic castration, gigantism, or change of host sex are all potential effects of microsporidian parasitism (in insects). In the most advanced cases of parasitism the microsporidium rules the host cell completely and controls its metabolism and reproduction, forming a xenoma.[11]
Replication takes place within the host's cells, which are infected by means of unicellular spores. These vary from 1–40 μm, making them some of the smallest eukaryotes.[citation needed] Microsporidia that infect mammals are 1.0–4.0 μm.[12] They also have the smallest eukaryotic genomes.
The terms "microsporidium" (pl. "microsporidia") and "microsporidian" are used as vernacular names for members of the group. The name Microsporidium Balbiani, 1884[13] is also used as a catchall genus for incertae sedis members.[14]

Morphology

Microsporidia lack mitochondria, instead possessing mitosomes. They also lack motile structures, such as flagella.
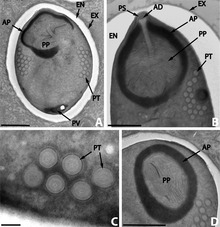
Microsporidia produce highly resistant spores, capable of surviving outside their host for up to several years. Spore morphology is useful in distinguishing between different species. Spores of most species are oval or pyriform, but rod-shaped or spherical spores are not unusual. A few genera produce spores of unique shape for the genus.
The spore is protected by a wall, consisting of three layers:
- an outer electron-dense exospore
- a median, wide and seemingly structureless endospore, containing chitin
- a thin internal plasma membrane
In most cases there are two closely associated nuclei, forming a diplokaryon, but sometimes there is only one.
The anterior half of the spore contains a harpoon-like apparatus with a long, thread-like polar filament, which is coiled up in the posterior half of the spore. The anterior part of the polar filament is surrounded by a polaroplast, a lamella of membranes. Behind the polar filament, there is a posterior vacuole.[11]
Infection
In the gut of the host the spore germinates; it builds up osmotic pressure until its rigid wall ruptures at its thinnest point at the apex. The posterior vacuole swells, forcing the polar filament to rapidly eject the infectious content into the cytoplasm of the potential host. Simultaneously the material of the filament is rearranged to form a tube which functions as a hypodermic needle and penetrates the gut epithelium.
Once inside the host cell, a
Medical implications
In animals and humans, microsporidia often cause chronic, debilitating diseases rather than lethal infections. Effects on the host include reduced longevity, fertility, weight, and general vigor. Vertical transmission of microsporidia is frequently reported.
In the case of insect hosts, vertical transmission often occurs as transovarial transmission, where the microsporidian parasites pass from the ovaries of the female host into eggs and eventually multiply in the infected larvae. Amblyospora salinaria n. sp. which infects the mosquito Culex salinarius Coquillett, and Amblyospora californica which infects the mosquito Culex tarsalis Coquillett, provide typical examples of transovarial transmission of microsporidia.[17][18][19][20] Microsporidia, specifically the mosquito-infecting Vavraia culicis, are being explored as a possible 'evolution-proof' malaria-control method.[21] Microsporidian infection of Anopheles gambiae (the principal vector of Plasmodium falciparum malaria) reduces malarial infection within the mosquito, and shortens the mosquito lifespan.[22] As the majority of malaria-infected mosquitoes naturally die before the malaria parasite is mature enough to transmit, any increase in mosquito mortality through microsporidian-infection may reduce malaria transmission to humans. In May 2020, researchers reported that Microsporidia MB, a symbiont in the midgut and ovaries of An. arabiensis, significantly impaired transmission of P. falciparum, had "no overt effect" on the fitness of host mosquitoes, and was transmitted vertically (through inheritance).[23]
Clinical
This section needs expansion. You can help by adding to it. (November 2013) |
Microsporidian infections of humans sometimes cause a disease called microsporidiosis. At least 14 microsporidian species, spread across eight genera, have been recognized as human pathogens. These include Trachipleistophora hominis.[24]
As hyperparasites
Microsporidia can infect a variety of hosts, including hosts which are themselves parasites. In that case, the microsporidian species is a
Genomes
Microsporidia have the smallest known (nuclear) eukaryotic
Horizontal gene transfer (HGT) seems to have occurred many times in microsporidia. For instance, the genomes of Encephalitozoon romaleae and Trachipleistophora hominis contain genes that derive from animals and bacteria, and some even from fungi.[27]
DNA repair
The Rad9-Rad1-Hus1 protein complex (also known as the 9-1-1 complex) in eukaryotes is recruited to sites of DNA damage where it is considered to help trigger the checkpoint-signaling cascade. Genes that code for heterotrimeric 9-1-1 are present in microsporidia.[28] In addition to the 9-1-1 complex, other components of the DNA repair machinery are also present indicting that repair of DNA damage likely occurs in microsporidia.[28]
Phylogeny
Phylogeny of Rozellomycota[29][30]
| Rozellomyceta |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Classification
The first described microsporidian genus,
Since the mid-2000s microsporidia are placed within the Fungi or as a sister-group of the Fungi with a common ancestor.[35][36][37][38]
Work to identify clades is largely based on habitat and host. Three classes of Microsporidia are proposed by Vossbrinck and Debrunner-Vossbrinck, based on the habitat: Aquasporidia, Marinosporidia and Terresporidia.[39]
A second classification by Cavalier-Smith 1993:[40]
- Subphyla Rudimicrospora Cavalier-Smith 1993
- Class Minisporea Cavalier-Smith 1993
- Order Minisporida Sprague, 1972
- Class MetchnikovelleaWeiser, 1977
- Order Metchnikovellida Vivier, 1975
- Class Minisporea Cavalier-Smith 1993
- Subphyla Polaroplasta Cavalier-Smith 1993
- Class Pleistophoridea Cavalier-Smith 1993
- Order Pleistophorida Stempell 1906
- Class Disporea Cavalier-Smith 1993
- Subclass Unikaryotia Cavalier-Smith 1993
- Subclass Diplokaryotia Cavalier-Smith 1993
- Class Pleistophoridea Cavalier-Smith 1993
|
See also
- List of Microsporidian genera
- Glugea, a genus of microsporidia
- Nosema apis, a microsporidian parasite of bees
References
- S2CID 249054641.
- ^ Balbiani, G (1882). "Sur les microsporidies ou psorospermies des Articulés". C. R. Acad. Sci. 95: 1168–71.
- ^ Delphy, J. 1936. Sous-règne des Protozoaires. In: Perrier, R. (ed.). La Faune de la France en tableaux synoptiques illustrés, vol 1A. Delagrave: Paris.
- PMID 6989987.
- ^ .
- ^ Sprague, V. (1977). Classification and phylogeny of the Microsporidia. In: Comparative pathobiology. vol. 2, Systematics of the Microsporidia. Lee A. Bulla & Thomas C. Cheng (ed.). pp. 1–30. New York: Plenum Press, [1].
- ^ a b Franzen, C. (2005). How do Microsporidia invade cells?. Folia Parasitologica, 52(1–2), 36–40. doi.org/10.14411/fp.2005.005
- S2CID 4686378.
- ISBN 978-2-9555841-0-1.
- .
- ^ a b Ronny Larsson, Lund University (Department of Cell and Organism Biology) Cytology and taxonomy of the microsporidia Archived 2009-09-12 at the Wayback Machine 2004.
- PMID 15777637.
- ^ Balbiani, G. 1884. Les Psorospermies des Articulés ou Microsporidies, pp. 150-168, 184. In: Leçons sur les sporozoaires. Paris: Doin, [2].
- ^ Hoffman, G. (1999). Parasites of North American Freshwater Fishes, 2nd edn, University of California Press, Berkeley, California, USA, p. 89, [3].
- PMID 24934702.
- PMID 17394631.
- PMID 536933.
- PMID 536610.
- ^ Jahn GC, Hall DW, Zam SG (1986). "A comparison of the life cycles of two Amblyospora (Microspora: Amblyosporidae) in the mosquitoes Culex salinarius and Culex tarsalis Coquillett". Journal of the Florida Anti-Mosquito Association. 57 (1): 24–27.
- PMID 9538031.
- PMID 19289199.
- PMID 19277119.
- PMID 32366903.
- PMID 23133373.
- ^ PMID 25174849.

- S2CID 246443154.

- ^ S2CID 24504235.
- ^ PMID 35439302.
- ^ ISSN 2077-7019.
- .
- ^ Nägeli, C. von (1857). "Über die neue Krankheit der Seidenraupe und verwandte Organismen. pp. 760–61. In: Caspary, R. (ed.). Bericht über die Verhandlungen der 33. Versammlung deutscher Naturforscher und Aerzte, gehalten in Bonn von 18 bis 24 September 1857". Botanische Zeitung. 15: 749–776.
- PMID 12142484.
- PMID 8302218.
- PMID 15590811.
- PMID 15923129.
- PMID 17010206.
- PMID 16626896.
- PMID 18976912.
- PMID 16004372.
- PMID 8302218.
- ISBN 9785020262249.
- S2CID 249054641.
External links
 Data related to Microsporidia at Wikispecies
Data related to Microsporidia at Wikispecies- BioHealthBase Bioinformatics Resource Center Database of microspordia sequences and related information.
- Microsporidia at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
