Mothers against decapentaplegic homolog 4
Ensembl | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| UniProt | |||||||||
| RefSeq (mRNA) | |||||||||
| RefSeq (protein) | |||||||||
| Location (UCSC) | Chr 18: 51.03 – 51.09 Mb | Chr 18: 73.77 – 73.84 Mb | |||||||
| PubMed search | [3] | [4] | |||||||
| View/Edit Human | View/Edit Mouse |
SMAD4, also called SMAD family member 4, Mothers against decapentaplegic homolog 4, or DPC4 (Deleted in Pancreatic Cancer-4) is a highly conserved protein present in all
SMAD 4 belongs to the
SMAD4 interacts with R-Smads, such as
Gene
In mammals, SMAD4 is coded by a gene located on chromosome 18. In humans, the SMAD4 gene contains 54 829 base pairs and is located from pair n° 51,030,212 to pair 51,085,041 in the region 21.1 of the chromosome 18.[7][8]

Protein
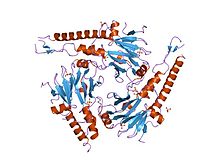
SMAD4 is a 552 amino-acid
.
The complex of two SMAD3 (or of two SMAD2) and one SMAD4 binds directly to DNA though interactions of their MH1 domains. These complexes are recruited to sites throughout the genome by cell lineage-defining transcription factors (LDTFs) that determine the context-dependent nature of TGF-β action. Early insights into the DNA binding specificity of Smad proteins came from oligonucleotide binding screens, which identified the palindromic duplex 5'–GTCTAGAC–3' as a high affinity binding sequence for SMAD3 and SMAD4 MH1 domains.[9] Other motifs have also been identified in promoters and enhancers. These additional sites contain the CAGCC motif and the GGC(GC)|(CG) consensus sequences, the latter also known as 5GC sites.[10] The 5GC-motifs are highly represented as clusters of sites, in SMAD-bound regions genome-wide. These clusters can also contain CAG(AC)|(CC) sites. SMAD3/SMAD4 complex also binds to the TPA-responsive gene promoter elements, which have the sequence motif TGAGTCAG.[11]
Structures
MH1 domain complexes with DNA motifs
The first structure of SMAD4 bound to DNA was the complex with the palindromic GTCTAGAC motif.



MH2 domain complexes
The MH2 domain, corresponding to the
Nomenclature and origin of name
SMADs are highly conserved across species, especially in the
Function and action mechanism
SMAD4 is a protein defined as an essential effector in the SMAD pathway. SMAD4 serves as a mediator between extracellular growth factors from the TGFβ family and genes inside the cell nucleus. The abbreviation co in co-SMAD stands for common mediator. SMAD4 is also defined as a signal transducer.
In the TGF-β pathway, TGF-β dimers are recognized by a transmembrane receptor, known as type II receptor. Once the type II receptor is activated by the binding of TGF-β, it phosphorylates a type I receptor. Type I receptor is also a
SMAD4 is a substrate of the
In the nucleus the heteromeric complex binds promoters and interact with transcriptional activators.
Many TGFβ ligands use this
Clinical significance
Genetic experiments such as
It has been shown that, in mouse KO of SMAD4, the
Deletions in the genes coding for SMAD1 and
SMAD4, is often found mutated in many cancers. The mutation can be inherited or acquired during an individual's lifetime. If inherited, the mutation affects both
Somatic mutations found in human cancers of the MH1 domain of SMAD 4 have been shown to inhibit the DNA-binding function of this domain.
SMAD 4 is also found mutated in the
Mutations in SMAD4 (mostly substitutions) can cause Myhre syndrome, a rare inherited disorder characterized by mental disabilities, short stature, unusual facial features, and various bone abnormalities.[26][27]
References
- ^ a b c GRCh38: Ensembl release 89: ENSG00000141646 – Ensembl, May 2017
- ^ a b c GRCm38: Ensembl release 89: ENSMUSG00000024515 – Ensembl, May 2017
- ^ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- PMID 22992590.
- ^ PMID 9759503.
- ^ "SMAD4 SMAD family member 4". Entrez Gene.
- ^ a b "SMAD 4". The Genetics Home Reference Website.
- PMID 9660945.
- ^ PMID 29234012.
- S2CID 4393852.
- PMID 21724602.
- S2CID 4407819.
- PMID 7768443.
- ^ White M (26 September 2014). "Sonic Hedgehog, DICER, and the Problem With Naming Genes". Pacific Standard.
- S2CID 9326115.
- PMID 25373906.
- PMID 10636916.
- PMID 27308623.
- PMID 27308538.
- PMID 15702493.
- PMID 19108831.
- PMID 16513794.
- PMID 19819941.
- ISBN 0-7216-0187-1.
- ^ "Growth-Mental Deficiency Syndrome of Myhre". National Organization for rare disorders. Archived from the original on 2 April 2015. Retrieved 26 March 2015.
- S2CID 5294309.
Further reading
- Miyazono K (2000). "TGF-beta signaling by Smad proteins". Cytokine & Growth Factor Reviews. 11 (1–2): 15–22. PMID 10708949.
- Wrana JL, Attisano L (2000). "The Smad pathway". Cytokine & Growth Factor Reviews. 11 (1–2): 5–13. PMID 10708948.
- Verschueren K, Huylebroeck D (2000). "Remarkable versatility of Smad proteins in the nucleus of transforming growth factor-beta activated cells". Cytokine & Growth Factor Reviews. 10 (3–4): 187–99. PMID 10647776.
- Massagué J (1998). "TGF-beta signal transduction". Annual Review of Biochemistry. 67: 753–91. PMID 9759503.
- Klein-Scory S, Zapatka M, Eilert-Micus C, Hoppe S, Schwarz E, Schmiegel W, Hahn SA, Schwarte-Waldhoff I (2008). "High-level inducible Smad4-reexpression in the cervical cancer cell line C4-II is associated with a gene expression profile that predicts a preferential role of Smad4 in extracellular matrix composition". BMC Cancer. 7: 209. PMID 17997817.
- Kalo E, Buganim Y, Shapira KE, Besserglick H, Goldfinger N, Weisz L, Stambolsky P, Henis YI, Rotter V (December 2007). "Mutant p53 attenuates the SMAD-dependent transforming growth factor beta1 (TGF-beta1) signaling pathway by repressing the expression of TGF-beta receptor type II". Molecular and Cellular Biology. 27 (23): 8228–42. PMID 17875924.
- Aretz S, Stienen D, Uhlhaas S, Stolte M, Entius MM, Loff S, Back W, Kaufmann A, Keller KM, Blaas SH, Siebert R, Vogt S, Spranger S, Holinski-Feder E, Sunde L, Propping P, Friedl W (November 2007). "High proportion of large genomic deletions and a genotype phenotype update in 80 unrelated families with juvenile polyposis syndrome". Journal of Medical Genetics. 44 (11): 702–9. PMID 17873119.
- Ali S, Cohen C, Little JV, Sequeira JH, Mosunjac MB, Siddiqui MT (October 2007). "The utility of SMAD4 as a diagnostic immunohistochemical marker for pancreatic adenocarcinoma, and its expression in other solid tumors". Diagnostic Cytopathology. 35 (10): 644–8. S2CID 36682992.
- Milet J, Dehais V, Bourgain C, Jouanolle AM, Mosser A, Perrin M, Morcet J, Brissot P, David V, Deugnier Y, Mosser J (October 2007). "Common variants in the BMP2, BMP4, and HJV genes of the hepcidin regulation pathway modulate HFE hemochromatosis penetrance". American Journal of Human Genetics. 81 (4): 799–807. PMID 17847004.
- Salek C, Benesova L, Zavoral M, Nosek V, Kasperova L, Ryska M, Strnad R, Traboulsi E, Minarik M (July 2007). "Evaluation of clinical relevance of examining K-ras, p16 and p53 mutations along with allelic losses at 9p and 18q in EUS-guided fine needle aspiration samples of patients with chronic pancreatitis and pancreatic cancer". World Journal of Gastroenterology. 13 (27): 3714–20. PMID 17659731.
- Sebestyén A, Hajdu M, Kis L, Barna G, Kopper L (September 2007). "Smad4-independent, PP2A-dependent apoptotic effect of exogenous transforming growth factor beta 1 in lymphoma cells". Experimental Cell Research. 313 (15): 3167–74. PMID 17643425.
- Martin MM, Buckenberger JA, Jiang J, Malana GE, Knoell DL, Feldman DS, Elton TS (September 2007). "TGF-beta1 stimulates human AT1 receptor expression in lung fibroblasts by cross talk between the Smad, p38 MAPK, JNK, and PI3K signaling pathways". American Journal of Physiology. Lung Cellular and Molecular Physiology. 293 (3): L790–9. PMID 17601799.
- Levy L, Howell M, Das D, Harkin S, Episkopou V, Hill CS (September 2007). "Arkadia activates Smad3/Smad4-dependent transcription by triggering signal-induced SnoN degradation". Molecular and Cellular Biology. 27 (17): 6068–83. PMID 17591695.
- Grijelmo C, Rodrigue C, Svrcek M, Bruyneel E, Hendrix A, de Wever O, Gespach C (August 2007). "Proinvasive activity of BMP-7 through SMAD4/src-independent and ERK/Rac/JNK-dependent signaling pathways in colon cancer cells". Cellular Signalling. 19 (8): 1722–32. PMID 17478078.
- Sonegawa H, Nukui T, Li DW, Takaishi M, Sakaguchi M, Huh NH (July 2007). "Involvement of deterioration in S100C/A11-mediated pathway in resistance of human squamous cancer cell lines to TGFbeta-induced growth suppression". Journal of Molecular Medicine. 85 (7): 753–62. S2CID 15667203.
- Sheikh AA, Vimalachandran D, Thompson CC, Jenkins RE, Nedjadi T, Shekouh A, Campbell F, Dodson A, Prime W, Crnogorac-Jurcevic T, Lemoine NR, Costello E (June 2007). "The expression of S100A8 in pancreatic cancer-associated monocytes is associated with the Smad4 status of pancreatic cancer cells". Proteomics. 7 (11): 1929–40. S2CID 35648264.
- Popović Hadzija M, Korolija M, Jakić Razumović J, Pavković P, Hadzija M, Kapitanović S (April 2007). "K-ras and Dpc4 mutations in chronic pancreatitis: case series". Croatian Medical Journal. 48 (2): 218–24. PMID 17436386.
- Losi L, Bouzourene H, Benhattar J (May 2007). "Loss of Smad4 expression predicts liver metastasis in human colorectal cancer". Oncology Reports. 17 (5): 1095–9. PMID 17390050.
- Karlsson G, Blank U, Moody JL, Ehinger M, Singbrant S, Deng CX, Karlsson S (March 2007). "Smad4 is critical for self-renewal of hematopoietic stem cells". The Journal of Experimental Medicine. 204 (3): 467–74. PMID 17353364.
- Takano S, Kanai F, Jazag A, Ijichi H, Yao J, Ogawa H, Enomoto N, Omata M, Nakao A (March 2007). "Smad4 is essential for down-regulation of E-cadherin induced by TGF-beta in pancreatic cancer cell line PANC-1". Journal of Biochemistry. 141 (3): 345–51. PMID 17301079.
