Multicellular organism
A multicellular organism is an
Multicellular organisms arise in various ways, for example by
Evolutionary history
Occurrence
Multicellularity has evolved independently at least 25 times in
Loss of multicellularity
Loss of multicellularity occurred in some groups.
Cancer
Multicellular organisms, especially long-living animals, face the challenge of
Separation of somatic and germ cells
In some multicellular groups, which are called Weismannists, a separation between a sterile somatic cell line and a germ cell line evolved. However, Weismannist development is relatively rare (e.g., vertebrates, arthropods, Volvox), as a great part of species have the capacity for somatic embryogenesis (e.g., land plants, most algae, many invertebrates).[26][10]
Origin hypotheses
One hypothesis for the origin of multicellularity is that a group of function-specific cells aggregated into a slug-like mass called a grex, which moved as a multicellular unit. This is essentially what slime molds do. Another hypothesis is that a primitive cell underwent nucleus division, thereby becoming a coenocyte. A membrane would then form around each nucleus (and the cellular space and organelles occupied in the space), thereby resulting in a group of connected cells in one organism (this mechanism is observable in Drosophila). A third hypothesis is that as a unicellular organism divided, the daughter cells failed to separate, resulting in a conglomeration of identical cells in one organism, which could later develop specialized tissues. This is what plant and animal embryos do as well as colonial choanoflagellates.[27][28]
Because the first multicellular organisms were simple, soft organisms lacking bone, shell, or other hard body parts, they are not well preserved in the fossil record.
Until recently,
The evolution of multicellularity could have occurred in several different ways, some of which are described below:
The symbiotic theory
This theory suggests that the first multicellular organisms occurred from symbiosis (cooperation) of different species of single-cell organisms, each with different roles. Over time these organisms would become so dependent on each other that they would not be able to survive independently, eventually leading to the incorporation of their genomes into one multicellular organism.[32] Each respective organism would become a separate lineage of differentiated cells within the newly created species.[citation needed]
This kind of severely co-dependent symbiosis can be seen frequently, such as in the relationship between
The cellularization (syncytial) theory
This theory states that a single unicellular organism, with multiple
The colonial theory
The colonial theory of
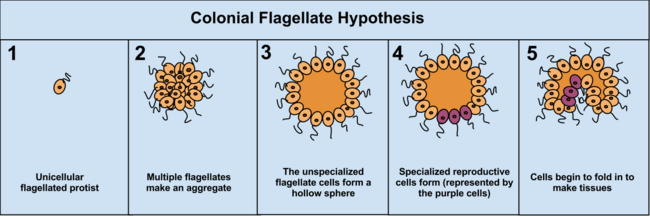
The advantage of the Colonial Theory hypothesis is that it has been seen to occur independently in 16 different protoctistan phyla. For instance, during food shortages the amoeba Dictyostelium groups together in a colony that moves as one to a new location. Some of these amoeba then slightly differentiate from each other. Other examples of colonial organisation in protista are Volvocaceae, such as Eudorina and Volvox, the latter of which consists of up to 500–50,000 cells (depending on the species), only a fraction of which reproduce.[36] For example, in one species 25–35 cells reproduce, 8 asexually and around 15–25 sexually. However, it can often be hard to separate colonial protists from true multicellular organisms, as the two concepts are not distinct; colonial protists have been dubbed "pluricellular" rather than "multicellular".[5]
The synzoospore theory
Some authors suggest that the origin of multicellularity, at least in Metazoa, occurred due to a transition from temporal to spatial
GK-PID
About 800 million years ago,[38] a minor genetic change in a single molecule called guanylate kinase protein-interaction domain (GK-PID) may have allowed organisms to go from a single cell organism to one of many cells.[39]
The role of viruses
Genes borrowed from
Oxygen availability hypothesis
This theory suggests that the oxygen available in the atmosphere of early Earth could have been the limiting factor for the emergence of multicellular life.[45] This hypothesis is based on the correlation between the emergence of multicellular life and the increase of oxygen levels during this time. This would have taken place after the Great Oxidation Event but before the most recent rise in oxygen. Mills[46] concludes that the amount of oxygen present during the Ediacaran is not necessary for complex life and therefore is unlikely to have been the driving factor for the origin of multicellularity.[citation needed]
Snowball Earth hypothesis
A snowball Earth is a geological event where the entire surface of the Earth is covered in snow and ice. The term can either refer to individual events (of which there were at least two) or to the larger geologic period during which all the known total glaciations occurred.
The most recent snowball Earth took place during the Cryogenian period and consisted of two global glaciation events known as the Sturtian and Marinoan glaciations. Xiao et al.[47] suggest that between the period of time known as the "Boring Billion" and the snowball Earth, simple life could have had time to innovate and evolve, which could later lead to the evolution of multicellularity.
The snowball Earth hypothesis in regards to multicellularity proposes that the Cryogenian period in Earth's history could have been the catalyst for the evolution of complex multicellular life. Brocks[48] suggests that the time between the Sturtian Glacian and the more recent Marinoan Glacian allowed for planktonic algae to dominate the seas making way for rapid diversity of life for both plant and animal lineages. Complex life quickly emerged and diversified in what is known as the Cambrian explosion shortly after the Marinoan.[citation needed]
Predation hypothesis
The predation hypothesis suggests that to avoid being eaten by predators, simple single-celled organisms evolved multicellularity to make it harder to be consumed as prey. Herron et al.[49] performed laboratory evolution experiments on the single-celled green alga, Chlamydomonas reinhardtii, using paramecium as a predator. They found that in the presence of this predator, C. reinhardtii does indeed evolve simple multicellular features.[citation needed]
Experimental evolution
It is impossible to know what happened when single cells evolved into multicellular organisms hundreds of millions of years ago. However, we can identify mutations that can turn single-celled organisms into multicellular ones. This would demonstrate the possibility of such an event. Unicellular species can relatively easily acquire mutations that make them attach to each other—the first step towards multicellularity. Multiple normally unicellular species have been evolved to exhibit such early steps:
- millimeters. Changes in multiple genes were identified. In addition, the authors reported that only anaerobic cultures of snowflake yeast evolved this trait, while the aerobic ones did not.[52]
- A range of green algae species have been experimentally evolved to form larger clumps. When Chlorella vulgaris is grown with a predator Ochromonas vallescia, it starts forming small colonies, which are harder to ingest due to the larger size. The same is true for Chlamydomonas reinhardtii under predation by Brachionus calyciflorus and Paramecium tetraurelia.
C. reinhartii normally starts as a motile single-celled propagule; this single cell asexually reproduces by undergoing 2–5 rounds of mitosis as a small clump of non-motile cells, then all cells become single-celled propagules and the clump dissolves. With a few generations under Paramecium predation, the "clump" becomes a persistent structure: only some cells become propagules. Some populations go further and evolved multi-celled propagules: instead of peeling off single cells from the clump, the clump now reproduces by peeling off smaller clumps.[53]
Advantages
Multicellularity allows an organism to exceed the size limits normally imposed by diffusion: single cells with increased size have a decreased surface-to-volume ratio and have difficulty absorbing sufficient nutrients and transporting them throughout the cell. Multicellular organisms thus have the competitive advantages of an increase in size without its limitations. They can have longer lifespans as they can continue living when individual cells die. Multicellularity also permits increasing complexity by allowing differentiation of cell types within one organism.[citation needed]
Whether all of these can be seen as advantages however is debatable: The vast majority of living organisms are single celled, and even in terms of biomass, single celled organisms are far more successful than animals, although not plants.[54] Rather than seeing traits such as longer lifespans and greater size as an advantage, many biologists see these only as examples of diversity, with associated tradeoffs.[citation needed]
Gene expression changes in the transition from uni- to multicellularity
During the evolutionary transition from unicellular organisms to multicellular organisms, the expression of genes associated with reproduction and survival likely changed.[55] In the unicellular state, genes associated with reproduction and survival are expressed in a way that enhances the fitness of individual cells, but after the transition to multicellularity, the pattern of expression of these genes must have substantially changed so that individual cells become more specialized in their function relative to reproduction and survival.[55] As the multicellular organism emerged, gene expression patterns became compartmentalized between cells that specialized in reproduction (germline cells) and those that specialized in survival (somatic cells). As the transition progressed, cells that specialized tended to lose their own individuality and would no longer be able to both survive and reproduce outside the context of the group.[55]
See also
- Bacterial colony
- Embryogenesis
- Organogenesis
- Unicellular organism
References
- ISBN 978-0-321-55418-5.
- ISBN 9780674975910.
- ^ PMID 25597443.
- ^ S. M. Miller (2010). "Volvox, Chlamydomonas, and the evolution of multicellularity". Nature Education. 3 (9): 65.
- ^ ISBN 978-0-7637-0066-9.
- S2CID 8060916.
- ^ .
- S2CID 13872783.
- S2CID 11961888.
- ^ PMID 24363320.
- ISSN 1093-4391. Archived from the original on March 8, 2012.)
{{cite journal}}: CS1 maint: unfit URL (link - ^ Margulis, L.; Chapman, M.J. (2009). Kingdoms and Domains: An illustrated guide to the phyla of life on Earth (4th ed.). Amsterdam, NL: Academic Press / Elsevier. p. 116.
- ^ Seravin, L. N. (2001). "The principle of counter-directional morphological evolution and its significance for constructing the megasystem of protists and other eukaryotes". Protistology. 2: 6–14.
- ^ Parfrey, L.W. & Lahr, D.J.G. (2013), p. 344.
- .
- ^ Seckbach, Joseph, Chapman, David J. [eds.]. (2010). Red algae in the genomic age. New York, NY, U.S.A.: Springer, p. 252, [1].
- PMID 20368268.
- ^ Richter, Daniel Joseph: The gene content of diverse choanoflagellates illuminates animal origins, 2013.
- ^ "Myxozoa". tolweb.org. Retrieved 14 April 2018.
- PMID 21301065.
- ^ Richter, D. J. (2013), p. 11.
- .
- ^ Lauckner, G. (1980). Diseases of protozoa. In: Diseases of Marine Animals. Kinne, O. (ed.). Vol. 1, p. 84, John Wiley & Sons, Chichester, UK.
- PMID 16590201.
- S2CID 4318184.
- ^ Ridley M (2004) Evolution, 3rd edition. Blackwell Publishing, p. 295–297.
- PMID 20971426.
- ^ Carroll, Sean B. (December 14, 2010). "In a Single-Cell Predator, Clues to the Animal Kingdom's Birth". The New York Times.
- ISBN 0-691-12029-3(paperback). An excellent book on the early history of life, very accessible to the non-specialist; includes extensive discussions of early signatures, fossilization, and organization of life.
- ^
El Albani, Abderrazak; et al. (1 July 2010). "Large colonial organisms with coordinated growth in oxygenated environments 2.1 Gyr ago". S2CID 4331375.
- S2CID 4448316.
- ISBN 978-0-465-07272-9. Archived from the originalon 2010-04-20. Retrieved 2017-09-05.
- ^
Hickman CP, Hickman FM (8 July 1974). Integrated Principles of Zoology (5th ed.). ISBN 978-0-8016-2184-0.
- S2CID 4385008.
- PMID 15714559.
- ^ AlgaeBase. Volvox Linnaeus, 1758: 820.
- S2CID 12795095. Archived from the original(PDF) on 2016-03-05.
- PMID 26554036.
- New York Times. Retrieved 7 January 2016.
- ^ PMID 27431520.
- ^ Letzter, Rafi (2018-02-02). "An Ancient Virus May Be Responsible for Human Consciousness". Live Science. Retrieved 2022-09-05.
- S2CID 4367889.
- PMID 24725407.
- ^ Slezak, Michael (2016), "No Viruses? No skin or bones either" (New Scientist, No. 2958, 1 March 2014) p.16
- S2CID 4200584.
- PMID 24550467.
- S2CID 90374085.
- S2CID 205258987.
- PMID 30787483.
- PMID 19013280.
- PMID 24145419.
- S2CID 236953093.
- PMID 30787483.
- ^
Bar-On, Yinon M.; Phillips, Rob; Milo, Ron (2018-06-19). "The biomass distribution on Earth". PMID 29784790.
- ^ a b c Grochau-Wright ZI, Nedelcu AM, Michod RE. The Genetics of Fitness Reorganization during the Transition to Multicellularity: The Volvocine regA-like Family as a Model. Genes (Basel). 2023 Apr 19;14(4):941. doi: 10.3390/genes14040941. PMID 37107699; PMCID: PMC10137558
External links
- Tree of Life Eukaryotes. Archived 2012-01-29 at the Wayback Machine.
