Negishi coupling
| Negishi coupling | |
|---|---|
| Named after | Ei-ichi Negishi |
| Reaction type | Coupling reaction |
| Identifiers | |
| Organic Chemistry Portal | negishi-coupling |
| RSC ontology ID | RXNO:0000088 |
The Negishi coupling is a widely employed
Palladium catalysts in general have higher
The Negishi coupling finds common use in the field of
The reaction is named after Ei-ichi Negishi who was a co-recipient of the 2010 Nobel Prize in Chemistry for the discovery and development of this reaction.
Negishi and coworkers originally investigated the cross-coupling of
Reaction mechanism
The reaction mechanism is thought to proceed via a standard Pd catalyzed cross-coupling pathway, starting with a Pd(0) species, which is oxidized to Pd(II) in an oxidative addition step involving the organohalide species.[8] This step proceeds with aryl, vinyl, alkynyl, and acyl halides, acetates, or triflates, with substrates following standard oxidative addition relative rates (I>OTf>Br>>Cl).[9]
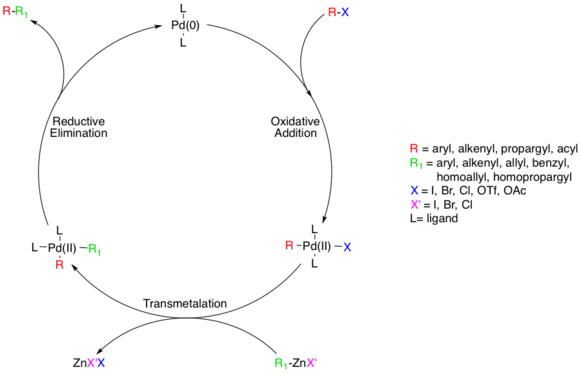
The actual mechanism of oxidative addition is unresolved, though there are two likely pathways. One pathway is thought to proceed via an

Though the additions are cis- the Pd(II) complex rapidly isomerizes to the trans- complex.[10]

Next, the transmetalation step occurs where the organozinc reagent exchanges its organic substituent with the halide in the Pd(II) complex, generating the trans- Pd(II) complex and a zinc halide salt. The organozinc substrate can be aryl, vinyl, allyl, benzyl, homoallyl, or homopropargyl.[8] Transmetalation is usually rate limiting and a complete mechanistic understanding of this step has not yet been reached though several studies have shed light on this process. It was recently determined that alkylzinc species must go on to form a higher-order zincate species prior to transmetalation whereas arylzinc species do not.[11] ZnXR and ZnR2 can both be used as reactive reagents, and Zn is known to prefer four coordinate complexes, which means solvent coordinated Zn complexes, such as ZnXR(solvent)2 cannot be ruled out a priori.[12] Studies indicate competing equilibriums exist between cis- and trans- bis alkyl organopalladium complexes, but that the only productive intermediate is the cis complex.[13][14]

The last step in the catalytic pathway of the Negishi coupling is reductive elimination, which is thought to proceed via a three coordinate transition state, yielding the coupled organic product and regenerating the Pd(0) catalyst. For this step to occur, the aforementioned cis- alkyl organopalladium complex must be formed.[15]

Both organozinc halides and diorganozinc compounds can be used as starting materials. In one model system it was found that in the transmetalation step the former give the cis-adduct R-Pd-R' resulting in fast reductive elimination to product while the latter gives the trans-adduct which has to go through a slow
A common side reaction is homocoupling. In one Negishi model system the formation of homocoupling was found to be the result of a second transmetalation reaction between the diarylmetal intermediate and arylmetal halide:[16]
- Ar–Pd–Ar' + Ar'–Zn–X → Ar'–Pd–Ar' + Ar–Zn–X
- Ar'–Pd–Ar' → Ar'–Ar' + Pd(0) (homocoupling)
- Ar–Zn–X + H2O → Ar–H + HO–Zn–X (reaction accompanied by dehalogenation)
Nickel catalyzed systems can operate under different mechanisms depending on the coupling partners. Unlike palladium systems which involve only Pd0 or PdII, nickel catalyzed systems can involve nickel of different oxidation states.[17] Both systems are similar in that they involve similar elementary steps: oxidative addition, transmetalation, and reductive elimination. Both systems also have to address issues of β-hydride elimination and difficult oxidative addition of alkyl electrophiles.[18]
For unactivated alkyl electrophiles, one possible mechanism is a transmetalation first mechanism. In this mechanism, the alkyl zinc species would first transmetalate with the nickel catalyst. Then the nickel would abstract the halide from the alkyl halide resulting in the alkyl radical and oxidation of nickel after addition of the radical.[19]

One important factor when contemplating the mechanism of a nickel catalyzed cross coupling is that reductive elimination is facile from NiIII species, but very difficult from NiII species. Kochi and Morrell provided evidence for this by isolating NiII complex Ni(PEt3)2(Me)(o-tolyl), which did not undergo reductive elimination quickly enough to be involved in this elementary step.[20]
Scope
The Negishi coupling has been applied the following illustrative syntheses:
- unsymmetrical 2-bromopyridine with tetrakis(triphenylphosphine)palladium(0),[21]
- biphenyl from o-tolylzinc chloride and o-iodotoluene and tetrakis(triphenylphosphine)palladium(0),[22]
- 5,7-hexadecadiene from 1-decyne and (Z)-1-hexenyl iodide.[23]
with hexaiodidobenzene, diferrocenylzinc and tris(dibenzylideneacetone)dipalladium(0) in tetrahydrofuran. The yield is only 4% signifying substantial crowding around the aryl core.
In a novel modification palladium is first oxidized by the
Recent conditions for the Negishi reaction have demonstrated extremely broad scope and tolerance of a broad range of functional groups and heteroaromatic nuclei and proceed at or near room temperature.[26]
Examples of nickel catalyzed Negishi couplings include sp2-sp2, sp2-sp3, and sp3-sp3 systems. In the system first studied by Negishi, aryl-aryl cross coupling was catalyzed by Ni(PPh3)4 generated in situ through reduction of Ni(acac)2 with PPh3 and (i-Bu)2AlH.[27]

Variations have also been developed to allow for the cross-coupling of aryl and alkenyl partners. In the variation developed by Knochel et al, aryl zinc bromides were reacted with vinyl triflates and vinyl halides.[28]

Reactions between sp3-sp3 centers are often more difficult; however, adding an unsaturated ligand with an electron withdrawing group as a cocatalyst improved the yield in some systems. It is believed that added coordination from the unsaturated ligand favors reductive elimination over β-hydride elimination.[29][30] This also works in some alkyl-aryl systems.[31]
Several asymmetric variants exist and many utilize Pybox ligands.[32][33][34]
Industrial applications
The Negishi coupling is not employed as frequently in industrial applications as its cousins the Suzuki reaction and Heck reaction, mostly as a result of the water and air sensitivity of the required aryl or alkyl zinc reagents.[35][36] In 2003 Novartis employed a Negishi coupling in the manufacture of PDE472, a phosphodiesterase type 4D inhibitor, which was being investigated as a drug lead for the treatment of asthma.[37] The Negishi coupling was used as an alternative to the Suzuki reaction providing improved yields, 73% on a 4.5 kg scale, of the desired benzodioxazole synthetic intermediate.[38]
Applications in total synthesis
Where the Negishi coupling is rarely used in industrial chemistry, a result of the aforementioned water and oxygen sensitivity, it finds wide use in the field of
(−)-stemoamide is a natural product found in the root extracts of ‘’Stemona tuberosa’’. These extracts have been used Japanese and Chinese
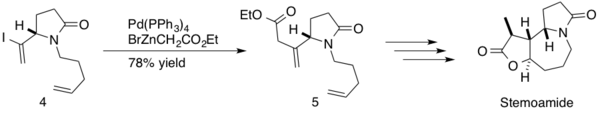
Kibayashi and coworkers utilized the Negishi coupling in the total synthesis of
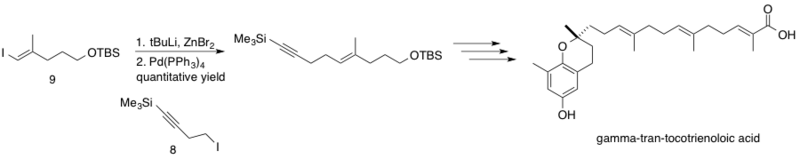
δ-trans-tocotrienoloic acid isolated from the plant, Chrysochlamys ulei, is a natural product shown to inhibit DNA polymerase β (pol β), which functions to repair DNA via base excision. Inhibition of pol B in conjunction with other chemotherapy drugs may increase the cytotoxicity of these chemotherapeutics, leading to lower effective dosages. The Negishi coupling was implemented in the synthesis of δ-trans-tocotrienoloic acid by Hecht and Maloney coupling the sp3 homopropargyl zinc reagent 8 with sp2 vinyl iodide 9.[44] The reaction proceeded with quantitative yield, coupling fragments mid-synthesis en route to the stereoselectively synthesized natural product δ-trans-tocotrienoloic acid.
Smith and Fu demonstrated that their method to couple secondary nucleophiles with secondary alkyl electrophiles could be applied to the formal synthesis of α-cembra-2,7,11-triene-4,6-diol, a target with antitumor activity. They achieved a 61% yield on a gram scale using their method to install an iso-propyl group. This method would be highly adaptable in this application for diversification and installing other alkyl groups to enable structure-activbity relationship (SAR) studies.[45]

Kirschning and Schmidt applied nickel catalyzed negishi cross-coupling to the first total synthesis of carolactone. In this application, they achieved 82% yield and dr = 10:1.[46]

Preparation of organozinc precursors
Alkylzinc reagents can be accessed from the corresponding alkyl bromides using iodine in dimethylacetamide (DMAC).[47] The catalytic I2 serves to activate the zinc towards nucleophilic addition.
Aryl zincs can be synthesized using mild reaction conditions via a
Organozincs can also be generated in situ and used in a one pot procedure as demonstrated by Knochel et al.[49]

See also
References
- .
- ISBN 978-0-12-429785-2.
- PMID 14640646.
- ISSN 0022-3263.
- PMID 16235910.
- .
- .
- ^ a b c Kurti L, Czako B (2005). Strategic Applications of Named Reactions in Organic Synthesis. New York: Elsevier Academic Press.
- ^ Andrew G Myers Research Group. "Chemistry 115 Handouts". Boston, Massachusetts: Harvard University Department of Chemistry.
- .
- PMID 22685029.
- PMID 23848308.
- ^ PMID 17328551.
- PMID 20623568.
- ^ Crabtree R (2005). The Organometallic Chemistry of the Transition Metals. Vol. 4. Hoboken, NJ: John Wiley and Sons Inc.
- S2CID 58240.
- PMID 19587955.
- PMID 14640646.
- PMID 25402209.
- ISSN 0002-7863.
- ^
Adam P. Smith, Scott A. Savage, J. Christopher Love, and Cassandra L. Fraser (2004). "Synthesis of 4-, 5-, and 6-methyl-2,2'-bipyridine by a Negishi cross-coupling strategy: 5-methyl-2,2'-bipyridine". Organic Syntheses
{{cite journal}}: CS1 maint: multiple names: authors list (link); Collected Volumes, vol. 10, p. 517. - ^
Ei-ichi Negishi, Tamotsu Takahashi, and Anthony O. King (1993). "Synthesis of biaryls via palladium-catalyzed cross-coupling: 2-methyl-4'-nitrobiphenyl". Organic Syntheses
{{cite journal}}: CS1 maint: multiple names: authors list (link); Collected Volumes, vol. 8, p. 430. - ^
Ei-ichi Negishi, Tamotsu Takahashi, and Shigeru Baba (1993). "Palladium-catalyzed synthesis of conjugated dienes". Organic Syntheses
{{cite journal}}: CS1 maint: multiple names: authors list (link); Collected Volumes, vol. 8, p. 295. - ^
Yu Y, Bond AD, Leonard PW, Lorenz UJ, Timofeeva TV, Vollhardt KP, Whitener GD, Yakovenko AA (June 2006). "Hexaferrocenylbenzene". Chemical Communications (24): 2572–4. PMID 16779481.
- ^
Zhao Y, Wang H, Hou X, Hu Y, Lei A, Zhang H, Zhu L (November 2006). "Oxidative cross-coupling through double transmetallation: surprisingly high selectivity for palladium-catalyzed cross-coupling of alkylzinc and alkynylstannanes". Journal of the American Chemical Society. 128 (47): 15048–9. PMID 17117830.
- PMID 23172689.
- ISSN 0022-3263.
- PMID 16235910.
- PMID 29710957.
- PMID 11777442.
- ISSN 0002-7863.
- PMID 15796523.
- PMID 18257579.
- PMID 17918274.
- PMID 22573393.
- PMID 18693766.
- .
- .
- PMID 15991198.
- doi:10.1071/ch03225.
- PMID 17451274.
- PMID 1336116.
- PMID 12153249.
- ^
Maloney DJ, Hecht SM (September 2005). "A stereocontrolled synthesis of delta-trans-tocotrienoloic acid". Organic Letters. 7 (19): 4297–300. PMID 16146411.
- PMID 18972493.
- PMID 22162345.
- ^
Huo S (February 2003). "Highly efficient, general procedure for the preparation of alkylzinc reagents from unactivated alkyl bromides and chlorides". Organic Letters. 5 (4): 423–5. PMID 12583734.
- .
- PMID 18693766.
External links
- The Negishi coupling at www.organic-chemistry.org







![Preparation of arylzinc reagent {\displaystyle {\begin{matrix}{}\\{\ce {Ar-I->[{\begin{matrix}{\ce {iPrMgCl}}\\{\text{THF}}\end{matrix}}][{\ce {ZnBr2}}]Ar-ZnBr}}\end{matrix}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/207cdded6086749bdb7e5dd64cf22c29306ff367)