Neisseria gonorrhoeae
| Neisseria gonorrhoeae | |
|---|---|
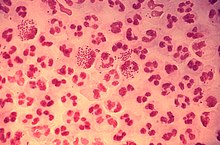
| |
extracellular bacteria. (CDC )
| |
| Scientific classification | |
| Domain: | Bacteria |
| Phylum: | Pseudomonadota |
| Class: | Betaproteobacteria |
| Order: | Neisseriales |
| Family: | Neisseriaceae |
| Genus: | Neisseria |
| Species: | N. gonorrhoeae
|
| Binomial name | |
| Neisseria gonorrhoeae | |
| Synonyms | |
Neisseria gonorrhoeae, also known as gonococcus (singular) or gonococci (plural), is a species of
It is oxidase positive and aerobic, and it survives phagocytosis and grows inside neutrophils.[4] Culturing it requires carbon dioxide supplementation and enriched agar (chocolate agar) with various antibiotics (Thayer–Martin). It exhibits antigenic variation through genetic recombination of its pili and surface proteins that interact with the immune system.[3]
Sexual transmission is through vaginal, anal, or oral sex.[5] Sexual transmission may be prevented through the use of barrier protection.[6] Perinatal transmission may occur during childbirth, and may be prevented by antibiotic treatment of the mother before birth and the application of antibiotic eye gel on the eyes of the newborn.[6] After an episode of gonococcal infection, infected persons do not develop immunity to future infections. Reinfection is possible due to N. gonorrhoeae's ability to evade the immune system by varying its surface proteins.[7]
N. gonorrhoeae can cause infection of the genitals, throat, and eyes.
Antibiotic resistance in N. gonorrhoeae is a growing public health concern, especially given its propensity to develop resistance easily.[13]
Microbiology
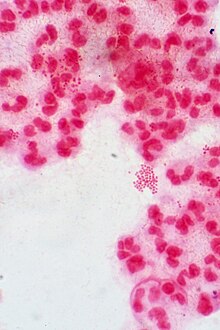
Culture and identification
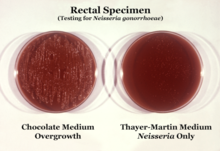

N. gonorrhoeae is usually isolated on Thayer–Martin agar (or VPN) agar in an atmosphere enriched with 3-7% carbon dioxide.[10] Thayer–Martin agar is a chocolate agar plate (heated blood agar) containing nutrients and antimicrobials (vancomycin, colistin, nystatin, and trimethoprim). This agar preparation facilitates the growth of Neisseria species while inhibiting the growth of contaminating bacteria and fungi. Martin Lewis and New York City agar are other types of selective chocolate agar commonly used for Neisseria growth.[10] N. gonorrhoeae is oxidase positive (possessing cytochrome c oxidase) and catalase positive (able to convert hydrogen peroxide to oxygen).[10] When incubated with the carbohydrates lactose, maltose, sucrose, and glucose, N. gonorrhoeae will oxidize only the glucose.[10]
Surface molecules
On its surface, N. gonorrhoeae bears hair-like
Dynamic
Lipooligosaccharide (LOS) is a low-weight version of lipopolysaccharide present on the surfaces of most other Gram-negative bacteria. It is a sugar (saccharide) side chain attached to lipid A (thus "lipo-") in the outer membrane coating the cell wall of the bacteria. The root "oligo" refers to the fact that it is a few sugars shorter than the typical lipopolysaccharide.[4] As an endotoxin, LOS provokes inflammation. The shedding of LOS by the bacteria is responsible for local injury in, for example, pelvic inflammatory disease.[4] Although its main function is as an endotoxin, LOS may disguise itself with host sialic acid and block initiation of the complement cascade.[4]
Antigenic variation
N. gonorrhoeae evades the immune system through a process called antigenic variation.[22] This process allows N. gonorrhoeae to recombine its genes and alter the antigenic determinants (sites where antibodies bind), such as the Type IV pili,[23] that adorn its surface.[4] Simply stated, the chemical composition of molecules is changed due to changes at the genetic level.[7] N. gonorrhoeae is able to vary the composition of its pili, and LOS; of these, the pili exhibit the most antigenic variation due to chromosomal rearrangement.[8][4] The PilS gene is an example of this ability to rearrange as its combination with the PilE gene is estimated to produce over 100 variants of the PilE protein.[7] These changes allow for adjustment to the differences in the local environment at the site of infection, evasion of recognition by targeted antibodies, and contribute to the lack of an effective vaccine.[7]
In addition to the ability to rearrange the genes it already has, it is also naturally competent to acquire new DNA (via plasmids), via its type IV pilus, specifically proteins Pil Q and Pil T.[24] These processes allow N. gonorrhoeae to acquire/spread new genes, disguise itself with different surface proteins, and prevent the development of immunological memory – an ability which has led to antibiotic resistance and has also impeded vaccine development.[25]
Phase variation
Phase variation is similar to antigenic variation, but instead of changes at the genetic level altering the composition of molecules, these genetic changes result in the turning on or off of a gene.[7] Phase variation most often arises from a frameshift in the expressed gene.[7] The Opacity, or Opa, proteins of N. gonorrhoeae rely strictly on phase variation.[7] Every time the bacteria replicate, they may switch multiple Opa proteins on or off through slipped-strand mispairing. That is, the bacteria introduce frameshift mutations that bring genes in or out of frame. The result is that different Opa genes are translated every time.[4] Pili are varied by antigenic variation, but also phase variation.[7] Frameshifts occur in both the pilE and pilC genes, effectively turning off the expression of pili in situations when they are not needed, such as after colonization when N. gonorrhoeae survives within cells as opposed to on their surfaces.[7]
Survival of gonococci
After gonococci invade and transcytose the host epithelial cells, they land in the submucosa, where neutrophils promptly consume them.[4] The pili and Opa proteins on the surface may interfere with phagocytosis,[8] but most gonococci end up in neutrophils. The exudates from infected individuals contain many neutrophils with ingested gonococci. Neutrophils release an oxidative burst of reactive oxygen species in their phagosomes to kill the gonococci.[26] However, a significant fraction of the gonococci can resist killing through the action of their catalase[4] which breaks down reactive oxygen species and is able to reproduce within the neutrophil phagosomes.[27]
Stohl and Seifert showed that the bacterial RecA protein, which mediates repair of DNA damage, plays an important role in gonococcal survival.[28] Michod et al. have suggested that N. gonorrhoeae may replace DNA damaged in neutrophil phagosomes with DNA from neighboring gonococci.[29] The process in which recipient gonococci integrate DNA from neighboring gonococci into their genome is called transformation.[30]

Genome
The genomes of several strains of N. gonorrhoeae have been sequenced. Most of them are about 2.1 Mb in size and encode 2,100 to 2,600 proteins (although most seem to be in the lower range).[31] For instance, strain NCCP11945 consists of one circular chromosome (2,232,025 bp) encoding 2,662 predicted open reading frames (ORFs) and one plasmid (4,153 bp) encoding 12 predicted ORFs. The estimated coding density over the entire genome is 87%, and the average G+C content is 52.4%, values that are similar to those of strain FA1090. The NCCP11945 genome encodes 54 tRNAs and four copies of 16S-23S-5S rRNA operons.[32]
Horizontal gene transfer
In 2011, researchers at Northwestern University found evidence of a human DNA fragment in a N. gonorrhoeae genome, the first example of horizontal gene transfer from humans to a bacterial pathogen.[33][34]
Disease
Symptoms
Symptoms of infection with N. gonorrhoeae differ depending on the site of infection and many infections are asymptomatic independent of sex.[35][15][5] It is important to note that depending on the route of transmission, N. gonorrhoeae may cause infection of the throat (pharyngitis) or infection of the anus/rectum (proctitis).[36][8]
Disseminated gonococcal infections can occur when N. gonorrhoeae enters the bloodstream, often spreading to the joints and causing a rash (dermatitis-arthritis syndrome).
Male
In symptomatic men, the primary symptom of genitourinary infection is urethritis – burning with urination (dysuria), increased urge to urinate, and a pus-like (purulent) discharge from the penis. The discharge may be foul smelling.[36] If untreated, scarring of the urethra may result in difficulty urinating. Infection may spread from the urethra in the penis to nearby structures, including the testicles (epididymitis/orchitis), or to the prostate (prostatitis).[36][8][38] Men who have had a gonorrhea infection have a significantly increased risk of having prostate cancer.[39]
Female
In symptomatic women, the primary symptoms of genitourinary infection are increased vaginal discharge, burning with urination (
Neonates (perinatal infection)
In
Transmission
N. gonorrhoeae is transmitted through vaginal, oral, or anal sex; nonsexual transmission is unlikely in adult infection.[5] It can also be transmitted to the newborn during passage through the birth canal if the mother has untreated genitourinary infection. Given the high rate of asymptomatic infection, all pregnant women should be tested for gonorrhea infection.[5] However, communal baths, towels or fabric, rectal thermometers and caregivers hands have been implicated as means of transmission in the pediatric setting.[40] Kissing has also been implicated as a theoretical means of transmission in the gay male population, based on newer studies.[41][42][43]
Traditionally, the bacterium was thought to move attached to spermatozoa, but this hypothesis did not explain female to male transmission of the disease. A recent study suggests that rather than "surf" on wiggling sperm, N. gonorrhoeae bacteria use pili to anchor onto proteins in the sperm and move through coital liquid.[44]
Infection
For N. gonorrhoeae, the first step after successful transmission is adherence to the epithelial cells found at the mucosal site that is infected.[45] The bacterium relies on type IV pili that attach and retract, pulling N. gonorrhoeae toward the epithelial membrane where its surface proteins, such as opacity proteins, can interact directly.[45] After adherence, N. gonorrhoeae replicates itself and forms microcolonies.[46] While colonizing, N. gonorrhoeae has the potential to transcytose across the epithelial barrier and work its way in to the bloodstream.[13] During growth and colonization, N. gonorrhoeae stimulates the release of cytokines and chemokines from host immune cells that are pro-inflammatory.[13] These pro-inflammatory molecules result in the recruitment of macrophages and neutrophils.[7] These phagocytic cells typically take in foreign pathogens and destroy them, but N. gonorrhoeae has evolved many mechanisms that allow it to survive within these immune cells and thwart the attempts at elimination.[7]
Prevention
Transmission is reduced by using latex barriers (e.g. condoms or dental dams) during sex and by limiting sexual partners.[6] Condoms and dental dams should be used during oral and anal sex, as well. Spermicides, vaginal foams, and douches are not effective for prevention of transmission.[4]
Treatment
The current treatment recommended by the CDC is an injected single dose of ceftriaxone (a third-generation cephalosporin).[47] Sexual partners (defined by the CDC as sexual contact within the past 60 days)[11] should also be notified, tested, and treated.[6][47] It is important that if symptoms persist after receiving treatment of N. gonorrhoeae infection, a reevaluation should be pursued.[47]
Antibiotic resistance
Antibiotic resistance in gonorrhea has been noted beginning in the 1940s. Gonorrhea was treated with penicillin, but doses had to be progressively increased to remain effective. By the 1970s, penicillin- and tetracycline-resistant gonorrhea emerged in the Pacific Basin. These resistant strains then spread to Hawaii, California, the rest of the United States, Australia and Europe. Fluoroquinolones were the next line of defense, but soon resistance to this antibiotic emerged, as well. Since 2007, standard treatment has been third-generation cephalosporins, such as ceftriaxone, which are considered to be our "last line of defense".[48][49] Recently, a high-level ceftriaxone-resistant strain of gonorrhea called H041 was discovered in Japan. Lab tests found it to be resistant to high concentrations of ceftriaxone, as well as most of the other antibiotics tested. Within N. gonorrhoeae, genes exist that confer resistance to every single antibiotic used to cure gonorrhea, but thus far they do not coexist within a single gonococcus. However, because of N. gonorrhoeae's high affinity for horizontal gene transfer, antibiotic-resistant gonorrhea is seen as an emerging public health threat.[49]
Serum resistance
As a Gram negative bacteria, N. gonorrhoeae requires defense mechanisms to protect itself against the complement system (or complement cascade), whose components are found with human serum.[15] There are three different pathways that activate this system however, they all result in the activation of complement protein 3 (C3).[50] A cleaved portion of this protein, C3b, is deposited on pathogenic surfaces and results in opsonization as well as the downstream activation of the membrane attack complex.[50] N. gonorrhoeae has several mechanisms to avoid this action.[13] As a whole, these mechanisms are referred to as serum resistance.[13]
History
Name origin
Neisseria gonorrhoeae is named for Albert Neisser, who isolated it as the causative agent of the disease gonorrhea in 1878.[13][3] Galen (130 AD) coined the term "gonorrhea" from the Greek gonos which means "seed" and rhoe which means "flow".[51][7] Thus, gonorrhea means "flow of seed", a description referring to the white penile discharge, assumed to be semen, seen in male infection.[13]
Discovery
In 1878, Albert Neisser isolated and visualized N. gonorrhoeae diplococci in samples of pus from 35 men and women with the classic symptoms of genitourinary infection with gonorrhea – two of whom also had infections of the eyes.[7] In 1882, Leistikow and Loeffler were able to grow the organism in culture.[13] Then in 1883, Max Bockhart proved conclusively that the bacterium isolated by Albert Neisser was the causative agent of the disease known as gonorrhea by inoculating the penis of a healthy man with the bacteria.[7] The man developed the classic symptoms of gonorrhea days after, satisfying the last of Koch's postulates. Until this point, researchers debated whether syphilis and gonorrhea were manifestations of the same disease or two distinct entities.[52][7] One such 18th-century researcher, John Hunter, tried to settle the debate in 1767[7] by inoculating a man with pus taken from a patient with gonorrhea. He erroneously concluded that both syphilis and gonorrhea were indeed the same disease when the man developed the copper-colored rash that is classic for syphilis.[50][52] Although many sources repeat that Hunter inoculated himself,[50][13] others have argued that it was in fact another man.[53] After Hunter's experiment other scientists sought to disprove his conclusions by inoculating other male physicians, medical students,[13] and incarcerated men with gonorrheal pus, who all developed the burning and discharge of gonorrhea. One researcher, Ricord, took the initiative to perform 667 inoculations of gonorrheal pus on patients of a mental hospital, with zero cases of syphilis.[7][13] Notably, the advent of penicillin in the 1940s made effective treatments for gonorrhea available.[citation needed]
See also
References
- ^ Euzéby JP, Parte AC. "Genus Neisseria". List of Prokaryotic Names with Standing in Nomenclature (LPSN). Retrieved 7 July 2017.
- ^ Neisser A (1879). "Ueber eine der Gonorrhoe eigentümliche Micrococusform" [About a micrococus form peculiar to gonorrhea]. Centralblatt für die medizinischen Wissenschaften (in German). 17 (28): 497–500.
- ^ ISBN 978-1-4381-0159-0.
- ^ ISBN 978-0-8385-8529-0.[page needed]
- ^ a b c d "Detailed STD Facts - Gonorrhea". www.cdc.gov. 26 September 2017. Retrieved 7 December 2017.
- ^ a b c d e "2015 Sexually Transmitted Diseases Treatment Guidelines". CDC. Centers for Disease Control and Prevention, U.S. Department of Health & Human Services. Archived from the original on 22 December 2015.
- ^ PMID 28357376.
- ^ OCLC 871305336.[page needed]
- ^ "Final Recommendation Statement: Chlamydia and Gonorrhea: Screening". U.S. Preventive Services Task Force. Retrieved 7 December 2017.
- ^ PMID 18159523.
- ^ a b "Gonococcal Infections - 2015 STD Treatment Guidelines". 4 January 2018.
- OCLC 781681739.
- ^ PMID 29430011.
- S2CID 219701418.
- ^ PMID 15489357.
- S2CID 4425775.
- PMID 18416602.
- S2CID 24580638.
- PMID 18223089.
- PMID 30181126.
- ^ STI Awareness: Gonorrhea. Planned Parenthood Advocates of Arizona. 11 April 2011. Retrieved 31 August 2011.
- S2CID 21366517.
- PMID 21812841.
- PMID 25866700.
- S2CID 21854666.
- PMID 15784537.
- PMID 30627130.
- PMID 16936020.
- PMID 18295550.
- PMID 27825443.
- ^ "Neisseria gonorrhoeae genome statistics". Broad Institute. Retrieved 8 April 2017.
- PMID 18586945.
- PMID 22016852.
- PMID 21325040.
- PMID 22256336.
- ^ ISBN 978-0-8385-8529-0.[page needed]
- ^ a b c "Gonococcal Infections - 2015 STD Treatment Guidelines". www.cdc.gov. Retrieved 7 December 2017.
- ^ "Gonorrhea (the clap) Symptoms". std-gov.org. 2 April 2015.
- PMID 24986642.
- PMID 17961874.
- ^ Bever L (10 May 2019). "You May Not Need to Have Sex to Transmit Gonorrhea, New Study Suggests". ScienceAlert. The Washington Post.
- S2CID 149446915.
- PMID 35865738.
- PMID 24595372.
- ^ PMID 96134.
- PMID 17682045.
- ^ a b c "CDC - Gonorrhea Treatment". www.cdc.gov. 22 July 2021. Retrieved 11 April 2022.
- ^ "UK doctors advised gonorrhoea has turned drug resistant". BBC News. 10 October 2011.
- ^ a b STI Awareness: Antibiotic-Resistant Gonorrhea. Planned Parenthood Advocates of Arizona. 6 March 2012. Retrieved 6 March 2012.
- ^ a b c d Janeway Jr CA, Travers P, Walport M, Shlomchik MJ (2001). "The complement system and innate immunity". Immunobiology: The Immune System in Health and Disease. 5th Edition.
- ^ "gonorrhea | Origin and meaning of gonorrhea by Online Etymology Dictionary". www.etymonline.com. Retrieved 5 December 2017.
- ^ ISBN 978-93-5152-748-0.
- PMID 15937780.
External links
- Todar K. "Pathogenic Neisseriae: Gonorrhea, Neonatal Ophthalmia and Meningococcal Meningitis". Todar's Online Textbook of Bacteriology.
- Gonorrhea at eMedicine
- "Neisseria gonorrhoeae". NCBI Taxonomy Browser. 485.
