Neural circuit

A neural circuit is a population of
Neural circuits have inspired the design of
Early study
Early treatments of neural
Connections between neurons
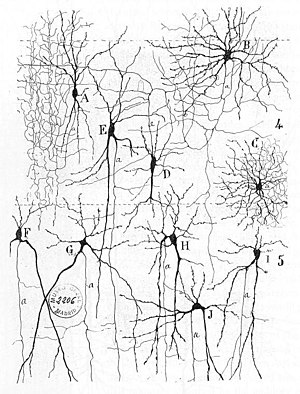
The connections between neurons in the brain are much more complex than those of the
The establishment of synapses enables the connection of neurons into millions of overlapping, and interlinking neural circuits. Presynaptic proteins called neurexins are central to this process.[5]
One principle by which neurons work is
On the
Connections display temporal and spatial characteristics. Temporal characteristics refers to the continuously modified activity-dependent efficacy of synaptic transmission, called spike-timing-dependent plasticity. It has been observed in several studies that the synaptic efficacy of this transmission can undergo short-term increase (called facilitation) or decrease (depression) according to the activity of the presynaptic neuron. The induction of long-term changes in synaptic efficacy, by long-term potentiation (LTP) or depression (LTD), depends strongly on the relative timing of the onset of the excitatory postsynaptic potential and the postsynaptic action potential. LTP is induced by a series of action potentials which cause a variety of biochemical responses. Eventually, the reactions cause the expression of new receptors on the cellular membranes of the postsynaptic neurons or increase the efficacy of the existing receptors through phosphorylation.
Backpropagating action potentials cannot occur because after an action potential travels down a given segment of the axon, the
A neuron in the brain requires a single signal to a
While in synapses in the
Circuitry
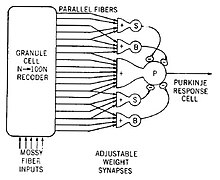
An example of a neural circuit is the trisynaptic circuit in the hippocampus. Another is the Papez circuit linking the hypothalamus to the limbic lobe. There are several neural circuits in the cortico-basal ganglia-thalamo-cortical loop. These circuits carry information between the cortex, basal ganglia, thalamus, and back to the cortex. The largest structure within the basal ganglia, the striatum, is seen as having its own internal microcircuitry.[6]
Neural circuits in the spinal cord called central pattern generators are responsible for controlling motor instructions involved in rhythmic behaviours. Rhythmic behaviours include walking, urination, and ejaculation. The central pattern generators are made up of different groups of spinal interneurons.[7]
There are four principal types of neural circuits that are responsible for a broad scope of neural functions. These circuits are a diverging circuit, a converging circuit, a reverberating circuit, and a parallel after-discharge circuit.[8]
In a diverging circuit, one neuron synapses with a number of postsynaptic cells. Each of these may synapse with many more making it possible for one neuron to stimulate up to thousands of cells. This is exemplified in the way that thousands of muscle fibers can be stimulated from the initial input from a single motor neuron.[8]
In a converging circuit, inputs from many sources are converged into one output, affecting just one neuron or a neuron pool. This type of circuit is exemplified in the respiratory center of the brainstem, which responds to a number of inputs from different sources by giving out an appropriate breathing pattern.[8]
A reverberating circuit produces a repetitive output. In a signalling procedure from one neuron to another in a linear sequence, one of the neurons may send a signal back to initiating neuron. Each time that the first neuron fires, the other neuron further down the sequence fire again sending it back to the source. This restimulates the first neuron and also allows the path of transmission to continue to its output. A resulting repetitive pattern is the outcome that only stops if one or more of the synapses fail, or if an inhibitory feed from another source causes it to stop. This type of reverberating circuit is found in the respiratory center that sends signals to the
In a parallel after-discharge circuit, a neuron inputs to several chains of neurons. Each chain is made up of a different number of neurons but their signals converge onto one output neuron. Each synapse in the circuit acts to delay the signal by about 0.5 msec, so that the more synapses there are, the longer is the delay to the output neuron. After the input has stopped, the output will go on firing for some time. This type of circuit does not have a feedback loop as does the reverberating circuit. Continued firing after the stimulus has stopped is called after-discharge. This circuit type is found in the reflex arcs of certain reflexes.[8]
Study methods
Different
The modern balance between the connectionist approach and the single-cell approach in
Clinical significance
Sometimes neural circuitries can become pathological and cause problems such as in
See also
- Feedback
- List of regions in the human brain
- Network science
- Neural coding
- Neural engineering
- Neural oscillation
- Pulse-coupled networks
- Systems neuroscience
- Connectomics
- Nerve tract
- Neural pathway
- Nerve plexus
References
- ISBN 9780878936953.
- ^ "Neural Circuits | Centre of Excellence for Integrative Brain Function". Centre of Excellence for Integrative Brain Function. 13 June 2016. Retrieved 4 June 2018.
- ^ Michael S. C. Thomas; James L. McClelland. "Connectionist models of cognition" (PDF). Stanford University. Archived from the original (PDF) on 2015-09-06. Retrieved 2015-08-31.
- ^ J. Y. Lettvin; H. R. Maturana; W. S. McCulloch; W. H. Pitts (1959), "What the frog's eye tells the frog's brain.", Proc. Inst. Radio Engr., no. 47, pp. 1940–1951
- PMID 29100073.
- PMID 20438237.
- PMID 23403923.
- ^ ISBN 9780071222075.
- S2CID 17487970.
- PMID 15973409.
- PMID 30366739.
- PMID 29755338.
Further reading
- Intrinsic plasticity Robert H. Cudmore, Niraj S. Desai Scholarpedia 3(2):1363. doi:10.4249/scholarpedia.1363
External links
- Comparison of Neural Networks in the Brain and Artificial Neural Networks
- Lecture notes at MIT OpenCourseWare
- Computation in the Brain
- Biological Neural Network Toolbox - A free Matlab toolbox for simulating networks of several different types of neurons
- WormWeb.org: Interactive Visualization of the C. elegans Neural Network - C. elegans, a nematode with 302 neurons, is the only organism for whom the entire neural network has been uncovered. Use this site to browse through the network and to search for paths between any 2 neurons.
- Introduction to Neurons and Neuronal Networks, Neuroscience Online (electronic neuroscience textbook)
- Delaying Pulse Networks (Wave Interference Networks)