Neural oscillation


Neural oscillations, or brainwaves, are rhythmic or repetitive patterns of neural activity in the
Neural oscillations in humans were observed by researchers as early as 1924 (by
History
Richard Caton discovered electrical activity in the cerebral hemispheres of rabbits and monkeys and presented his findings in 1875.[4] Adolf Beck published in 1890 his observations of spontaneous electrical activity of the brain of rabbits and dogs that included rhythmic oscillations altered by light detected with electrodes directly placed on the surface of the brain.[5] Before Hans Berger, Vladimir Vladimirovich Pravdich-Neminsky published the first animal EEG and the evoked potential of a dog.[6]
Overview
Neural oscillations are observed throughout the central nervous system at all levels, and include
Neural oscillations have been most widely studied in neural activity generated by large groups of neurons. Large-scale activity can be measured by techniques such as EEG. In general, EEG signals have a broad spectral content similar to
Although neural oscillations in human brain activity are mostly investigated using EEG recordings, they are also observed using more invasive recording techniques such as
Neural oscillations are commonly studied from a mathematical framework and belong to the field of "neurodynamics", an area of research in the
The functions of neural oscillations are wide-ranging and vary for different types of oscillatory activity. Examples are the generation of rhythmic activity such as a heartbeat and the neural binding of sensory features in perception, such as the shape and color of an object. Neural oscillations also play an important role in many neurological disorders, such as excessive synchronization during seizure activity in epilepsy or tremor in patients with Parkinson's disease. Oscillatory activity can also be used to control external devices such as a brain–computer interface.[25]
Physiology
Oscillatory activity is observed throughout the central nervous system at all levels of organization. Three different levels have been widely recognized: the micro-scale (activity of a single neuron), the meso-scale (activity of a local group of neurons) and the macro-scale (activity of different brain regions).[26]
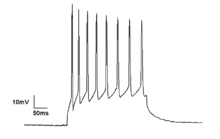
Microscopic
Neurons generate
Neuronal spiking can be classified by their activity patterns. The excitability of neurons can be subdivided in Class I and II. Class I neurons can generate action potentials with arbitrarily low frequency depending on the input strength, whereas Class II neurons generate action potentials in a certain frequency band, which is relatively insensitive to changes in input strength.[19] Class II neurons are also more prone to display sub-threshold oscillations in membrane potential.
Mesoscopic
A group of neurons can also generate oscillatory activity. Through synaptic interactions, the firing patterns of different neurons may become synchronized and the rhythmic changes in electric potential caused by their action potentials will add up (
Macroscopic
Neural oscillation can also arise from interactions between different brain areas coupled through the structural
Mechanisms
Neuronal properties
Scientists have identified some intrinsic
Network properties
Apart from intrinsic properties of neurons,
If a group of neurons engages in synchronized oscillatory activity, the neural ensemble can be mathematically represented as a single oscillator.
Neuromodulation
In addition to fast direct
Mathematical description
Oscillations can often be described and analyzed using mathematics. Mathematicians have identified several
Single neuron model

A model of a biological neuron is a mathematical description of the properties of nerve cells, or neurons, that is designed to accurately describe and predict its biological processes. One of the most successful neuron models is the Hodgkin–Huxley model, for which
The Hodgkin–Huxley model is too complicated to understand using classical mathematical techniques, so researchers often turn to simplifications such as the
Spiking model
A neural network model describes a population of physically interconnected neurons or a group of disparate neurons whose inputs or signalling targets define a recognizable circuit. These models aim to describe how the dynamics of neural circuitry arise from interactions between individual neurons. Local interactions between neurons can result in the synchronization of spiking activity and form the basis of oscillatory activity. In particular, models of interacting
Neural mass model
Neural field models are another important tool in studying neural oscillations and are a mathematical framework describing evolution of variables such as mean firing rate in space and time. In modeling the activity of large numbers of neurons, the central idea is to take the density of neurons to the
Kuramoto model
The
Activity patterns
Both single neurons and groups of neurons can generate oscillatory activity spontaneously. In addition, they may show oscillatory responses to perceptual input or motor output. Some types of neurons will fire rhythmically in the absence of any synaptic input. Likewise, brain-wide activity reveals oscillatory activity while subjects do not engage in any activity, so-called resting-state activity. These ongoing rhythms can change in different ways in response to perceptual input or motor output. Oscillatory activity may respond by increases or decreases in frequency and amplitude or show a temporary interruption, which is referred to as phase resetting. In addition, external activity may not interact with ongoing activity at all, resulting in an additive response.
Ongoing activity
Spontaneous activity is
Ongoing brain activity may also have an important role in perception, as it may interact with activity related to incoming stimuli. Indeed,
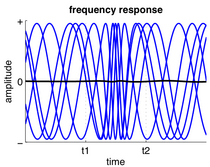
Frequency response
In response to input, a neuron or
Amplitude response
Next to evoked activity, neural activity related to stimulus processing may result in induced activity. Induced activity refers to modulation in ongoing brain activity induced by processing of stimuli or movement preparation. Hence, they reflect an indirect response in contrast to evoked responses. A well-studied type of induced activity is amplitude change in oscillatory activity. For instance,
Phase resetting
Phase resetting occurs when input to a neuron or neuronal ensemble resets the phase of ongoing oscillations.[62] It is very common in single neurons where spike timing is adjusted to neuronal input (a neuron may spike at a fixed delay in response to periodic input, which is referred to as phase locking[19]) and may also occur in neuronal ensembles when the phases of their neurons are adjusted simultaneously. Phase resetting is fundamental for the synchronization of different neurons or different brain regions[18][38] because the timing of spikes can become phase locked to the activity of other neurons.
Phase resetting also permits the study of evoked activity, a term used in electroencephalography and magnetoencephalography for responses in brain activity that are directly related to stimulus-related activity. Evoked potentials and event-related potentials are obtained from an electroencephalogram by stimulus-locked averaging, i.e. averaging different trials at fixed latencies around the presentation of a stimulus. As a consequence, those signal components that are the same in each single measurement are conserved and all others, i.e. ongoing or spontaneous activity, are averaged out. That is, event-related potentials only reflect oscillations in brain activity that are phase-locked to the stimulus or event. Evoked activity is often considered to be independent from ongoing brain activity, although this is an ongoing debate.[63][64]
Asymmetric amplitude modulation
It has recently been proposed that even if phases are not aligned across trials, induced activity may still cause event-related potentials because ongoing brain oscillations may not be symmetric and thus amplitude modulations may result in a baseline shift that does not average out.[65][66] This model implies that slow event-related responses, such as asymmetric alpha activity, could result from asymmetric brain oscillation amplitude modulations, such as an asymmetry of the intracellular currents that propagate forward and backward down the dendrites.[67] Under this assumption, asymmetries in the dendritic current would cause asymmetries in oscillatory activity measured by EEG and MEG, since dendritic currents in pyramidal cells are generally thought to generate EEG and MEG signals that can be measured at the scalp.[68]
Cross-frequency coupling
Cross-frequency coupling (CFC) describes the coupling (statistical correlation) between a slow wave and a fast wave. There are many kinds, generally written as A-B coupling, meaning the A of a slow wave is coupled with the B of a fast wave. For example, phase–amplitude coupling is where the phase of a slow wave is coupled with the amplitude of a fast wave.[69]
The theta-gamma code is a coupling between theta wave and gamma wave in the hippocampal network. During a theta wave, 4 to 8 non-overlapping neuron ensembles are activated in sequence. This has been hypothesized to form a neural code representing multiple items in a temporal frame [70][71]
Function
Neural synchronization can be modulated by task constraints, such as
Pacemaker
Cells in the
Central pattern generator
Synchronized firing of neurons also forms the basis of periodic motor commands for rhythmic movements. These rhythmic outputs are produced by a group of interacting neurons that form a network, called a
Information processing
Neuronal spiking is generally considered the basis for information transfer in the brain. For such a transfer, information needs to be coded in a spiking pattern. Different types of coding schemes have been proposed, such as
Temporal decoding
Single-cell intrinsic oscillators serve as valuable tools for decoding temporally-encoded sensory information. This information is encoded through inter-spike intervals, and intrinsic oscillators can act as 'temporal rulers' for precisely measuring these intervals. One notable mechanism for achieving this is the neuronal phase-locked loop (NPLL). In this mechanism, cortical oscillators undergo modulation influenced by the firing rates of thalamocortical 'phase detectors,' which, in turn, gauge the disparity between the cortical and sensory periodicity.[78]
Perception
Synchronization of neuronal firing may serve as a means to group spatially segregated neurons that respond to the same stimulus in order to bind these responses for further joint processing, i.e. to exploit temporal synchrony to encode relations. Purely theoretical formulations of the binding-by-synchrony hypothesis were proposed first,[79] but subsequently extensive experimental evidence has been reported supporting the potential role of synchrony as a relational code.[80]
The functional role of synchronized oscillatory activity in the brain was mainly established in experiments performed on awake kittens with multiple electrodes implanted in the visual cortex. These experiments showed that groups of spatially segregated neurons engage in synchronous oscillatory activity when activated by visual stimuli. The frequency of these oscillations was in the range of 40 Hz and differed from the periodic activation induced by the grating, suggesting that the oscillations and their synchronization were due to internal neuronal interactions.[80] Similar findings were shown in parallel by the group of Eckhorn, providing further evidence for the functional role of neural synchronization in feature binding.[81] Since then, numerous studies have replicated these findings and extended them to different modalities such as EEG, providing extensive evidence of the functional role of gamma oscillations in visual perception.
Gilles Laurent and colleagues showed that oscillatory synchronization has an important functional role in odor perception. Perceiving different odors leads to different subsets of neurons firing on different sets of oscillatory cycles.
Neural oscillations are also thought be involved in the
Motor coordination
Oscillations have been commonly reported in the motor system. Pfurtscheller and colleagues found a reduction in
Recently it was found that cortical oscillations propagate as
Oscillatory rhythms at 10 Hz have been recorded in a brain area called the
Memory
Neural oscillations, in particular
Sleep and consciousness
Sleep is a naturally recurring state characterized by reduced or absent consciousness and proceeds in cycles of rapid eye movement (REM) and non-rapid eye movement (NREM) sleep. Sleep stages are characterized by spectral content of EEG: for instance, stage N1 refers to the transition of the brain from alpha waves (common in the awake state) to theta waves, whereas stage N3 (deep or slow-wave sleep) is characterized by the presence of delta waves.[106] The normal order of sleep stages is N1 → N2 → N3 → N2 → REM.[citation needed]
Development
Neural oscillations may play a role in neural development. For example, retinal waves are thought to have properties that define early connectivity of circuits and synapses between cells in the retina.[107]
Pathology
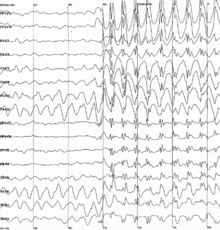
Specific types of neural oscillations may also appear in pathological situations, such as
Tremor
A tremor is an involuntary, somewhat rhythmic, muscle contraction and relaxation involving to-and-fro movements of one or more body parts. It is the most common of all involuntary movements and can affect the hands, arms, eyes, face, head, vocal cords, trunk, and legs. Most tremors occur in the hands. In some people, tremor is a symptom of another neurological disorder. Many different forms of tremor have been identified, such as essential tremor or Parkinsonian tremor. It is argued that tremors are likely to be multifactorial in origin, with contributions from neural oscillations in the central nervous systems, but also from peripheral mechanisms such as reflex loop resonances.[108]
Epilepsy
Epilepsy is a common chronic neurological disorder characterized by
Thalamocortical dysrhythmia
In thalamocortical dysrhythmia (TCD), normal thalamocortical resonance is disrupted. The thalamic loss of input allows the frequency of the thalamo-cortical column to slow into the theta or delta band as identified by MEG and EEG by machine learning.[110] TCD can be treated with neurosurgical methods like thalamotomy.
Applications
Clinical endpoints
Neural oscillations are sensitive to several drugs influencing brain activity; accordingly, biomarkers based on neural oscillations are emerging as secondary endpoints in clinical trials and in quantifying effects in pre-clinical studies. These biomarkers are often named "EEG biomarkers" or "Neurophysiological Biomarkers" and are quantified using quantitative electroencephalography (qEEG). EEG biomarkers can be extracted from the EEG using the open-source Neurophysiological Biomarker Toolbox.
Brain–computer interface
Neural oscillation has been applied as a control signal in various brain–computer interfaces (BCIs).[111] For example, a non-invasive BCI can be created by placing electrodes on the scalp and then measuring the weak electric signals. Although individual neuron activities cannot be recorded through non-invasive BCI because the skull damps and blurs the electromagnetic signals, oscillatory activity can still be reliably detected. The BCI was introduced by Vidal in 1973[112] as challenge of using EEG signals to control objects outside human body.
After the BCI challenge, in 1988, alpha rhythm was used in a brain rhythm based BCI for control of a physical object, a robot.[113][114] Alpha rhythm based BCI was the first BCI for control of a robot.[115][116] In particular, some forms of BCI allow users to control a device by measuring the amplitude of oscillatory activity in specific frequency bands, including mu and beta rhythms.
Examples
A non-inclusive list of types of oscillatory activity found in the central nervous system:
- Alpha wave
- Beta wave
- Bursting
- Cardiac cycle
- Delta wave
- Epileptic seizure
- Gamma wave
- Mathematical modeling of electrophysiological activity in epilepsy
- Mu wave
- PGO waves
- Thalamocortical oscillations
- Sharp wave–ripple complexes
- Sleep spindle
- Subthreshold membrane potential oscillations
- Theta wave
See also
- Cybernetics
- Dynamical systems theory
- EEG analysis
- Neurocybernetics
- Oscillatory neural network
- Systems neuroscience
- ThetaHealing
- Phase resetting in neurons
References
- ^ license.
- ^ a b c Ahissar, E. & Vaadia, E. Oscillatory activity of single units in a somatosensory cortex of an awake monkey and their possible role in texture analysis. Proc Natl Acad Sci USA 87, 8935-8939 (1990).
- PMID 25408634.
- ^ "Caton, Richard - The electric currents of the brain". echo.mpiwg-berlin.mpg.de. Retrieved 2018-12-21.
- S2CID 205664545.
- ^ Pravdich-Neminsky VV (1913). "Ein Versuch der Registrierung der elektrischen Gehirnerscheinungen". Zentralblatt für Physiologie. 27: 951–60.
- ^ S2CID 6275292.
- S2CID 7422401.
- ^ S2CID 2749709.
- S2CID 34728448.
- PMID 28537480.
- ^ PMID 34429378.
- ^ S2CID 208614924.
- S2CID 10835361.
- ^ S2CID 34728448.
- PMID 13480240.
- S2CID 11922975.
- ^ S2CID 18651043.
- ^ a b c Izhikevich EM (2007). Dynamical systems in neuroscience. Cambridge, Massachusetts: The MIT Press.
- ^ PMID 3795074.
- PMID 18160427.
- S2CID 121438105.
- S2CID 2811608.
- PMID 3281253.
- PMID 27959736.
- ^ ISBN 978-3-540-58967-9.
- ^ PMID 20664082.
- ISBN 9780195027969.
- PMID 19396156.
- PMID 9854256.
- PMID 21451032.
- PMID 11742683.
- PMID 24321555.
- PMID 3059497.
- PMID 1992481.
- ^ Ahissar, E., Haidarliu, S., and Zacksenhouse, M. (1997). Decoding temporally encoded sensory input by cortical oscillations and thalamic phase comparators. Proc Natl Acad Sci USA 94, 11633-11638.
- PMID 19658549.
- ^ ISBN 978-0-521-53352-2.
- ^ Andrea Brovelli, Steven L. Bressler and their colleagues, 2004
- PMID 19416820.
- PMID 18421835.
- S2CID 8002293.
- PMID 23055474.
- ^ Lapicque, LM (1907). "Recherches quantitatives sur l'excitation electrique des nerfs". J Physiol Paris. 9: 620–635.
- S2CID 46170924.
- PMID 11102670.
- PMID 26578893.
- S2CID 8751526.
- ^ Bressloff PC, Cowan JD (2003) Spontaneous pattern formation in primary visual cortex. In: J Hogan, AR Krauskopf, M di Bernado, RE Wilson (Eds.), Nonlinear dynamics and chaos: where do we go from here?
- ^ Kuramoto Y (1984). Chemical Oscillations, Waves, and Turbulence. Dover Publications.
- ^ Ermentrout B (1994). "An introduction to neural oscillators". In F Ventriglia (ed.). Neural Modeling and Neural Networks. pp. 79–110.
- PMID 21151358.
- S2CID 13959959.
- PMID 19571142.
- S2CID 15979590.
- PMID 12958209.
- PMID 19261866.
- PMID 19535598.
- PMID 18287498.
- S2CID 1308261.
- ^ S2CID 24756702.
- ISBN 978-3-540-65697-5.
- S2CID 15200185.
- S2CID 16210275.
- S2CID 12113334.
- PMID 18667610.
- PMID 21060804.
- .
- S2CID 3545001.
- PMID 23522038.
- PMID 31263399.
- ^ PMID 8466179.
- PMID 7605074.
- S2CID 1294374.
- S2CID 102514.
- PMID 25582580.
- ^ PMID 27976720.
- ^ Ahissar, E., Nelinger, G., Assa, E., Karp, O. & Saraf-Sinik, I. Thalamocortical loops as temporal demodulators across senses. Communications Biology 6, 562 (2023).
- PMID 4445414.
- ^ S2CID 4281744.
- S2CID 206771651.
- S2CID 4286308.
- S2CID 10744144.
- S2CID 205024830.
- S2CID 4424801.
- S2CID 29616055.
- )
- PMID 21715631.
- PMID 67933.
- PMID 8985892.
- PMID 8506287.
- PMID 8788955.
- S2CID 2178927.
- PMID 9175005.
- PMID 26634293.
- PMID 29963631.
- S2CID 16430438.
- .
- PMID 24204220.
- PMID 660226.
- PMID 8271223.
- PMID 11854526.
- ^ Buszaki G (2006). Rhythms of the brain. Oxford University Press.
- PMID 20060015.
- S2CID 4417989.
- PMID 32332806.
- PMID 19580682.
- PMID 10908186.
- S2CID 13928602.
- PMID 29549239.
- PMID 17076808.
- PMID 4583653.
- S2CID 62179588.
- S2CID 60642344.
- doi:10.20388/OMP.003.0035 (inactive 31 January 2024).)
{{cite journal}}: CS1 maint: DOI inactive as of January 2024 (link - PMID 28275048.
Further reading
- Buzsáki G (2006). Rhythms of the Brain. Oxford University Press. ISBN 978-0-19-530106-9.
- Freeman W (1975). Mass Action in the Nervous System. Academic Press. ISBN 978-0124120471. Archived from the originalon 2015-07-05.