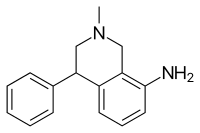
Nomifensine
 | |
| Clinical data | |
|---|---|
| Trade names | Merital |
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Elimination half-life | 1.5–4 hours |
| Excretion | Kidney (88%) within 24 hours[2] |
| Identifiers | |
| |
JSmol) | |
| |
| |
| | |
Nomifensine (Merital, Alival) is a
The drug was developed in the 1960s by
Some case reports in the 1980s suggested that there was potential for psychological dependence on nomifensine, typically in patients with a history of stimulant addiction, or when the drug was used in very high doses (400–600 mg per day).[9]
In a 1989 study it was investigated for use in treating adult ADHD and proven effective.[10] In a 1977 study it was not proven of benefit in advanced parkinsonism, except for depression associated with the parkinsonism.[11]
Clinical uses
Nomifensine was investigated for use as an antidepressant in the 1970s, and was found to be a useful antidepressant at doses of 50–225 mg per day, both motivating and anxiolytic.
Side effects and withdrawal from market
During treatment with nomifensine there were relatively few adverse effects, mainly renal failure, paranoid symptoms, drowsiness or insomnia, headache, and dry mouth. Side effects affecting the cardiovascular system included tachycardia and palpitations, but nomifensine was significantly less cardiotoxic than the standard tricyclic antidepressants.[12]
Due to a risk of
In 2012 structure-affinity relationship data (compare SAR) were published.[15]
Synthesis
Note that nomifensine was a Progenitor to Gastrophenazine.[16] See also: Isatin derivatives.[17]

The alkylation between N-methyl-2-nitrobenzylamine [56222-08-3] (1) and
See also
- Amineptine
- Diclofensine
- Perafensine
- The combination of Clobazam / nomifensine is called Psyton [75963-47-2].[27]
References
- ^ Anvisa (2023-03-31). "RDC Nº 784 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial" [Collegiate Board Resolution No. 784 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Diário Oficial da União (published 2023-04-04). Archived from the original on 2023-08-03. Retrieved 2023-08-16.
- PMID 6370971.
- S2CID 23952170.
- ^ 'Chirality and Biological Activity of Drugs' page 138
- ^ US patent 3577424, Ehrhart, Gustav; Schmitt, Karl & Hoffmann, Irmgard et al., "4-Phenyl-8-Amino Tetrahydroisoquinolines", issued 1971-05-04, assigned to Farbwerke Hoechst
- PMID 334230.
- PMID 6370985.
- PMID 20742679.
- S2CID 29192368.
- S2CID 1932119.
- PMID 334223.
- PMID 911653.
- ^ "Nomifensine DB04821". Drugbank.ca.
- PMID 3058454.
- PMID 23084899.
- ^ PMID 3806569.
- ^ DE3333994 idem Karl-Heinz Boltze, et al. U.S. patent 4,564,613 (1986 to TROPONWERKE & Co KG A CORP OF GERMANY GmbH, Troponwerke GmbH).
- PMID 5109496.
- ^ GB 1164192, "Tetrahydroisoquinolines and process for preparing them", published 1969-09-17 corresp to G. Ehrhart et al., U.S. patent 3,577,424 (1969, 1971, both to Hoechst AG
- ^ Gustav Dipl Chem Dr Ehrhart, et al. DE 1795829 (1977 to Hoechst AG).
- ^ Orchideja Bontscheva Sabunova, et al. EP 0066885 (1982 to Dso "pharmachim").
- ^ Ulin, J.; Gee, A.D.; Malmborg, P.; Tedroff, J.; Långström, B. (1989). "Synthesis of racemic (+) and (−) N-[methyl-11C]nomifensine, a ligand for evaluation of monoamine re-uptake sites by use of positron emission tomography". International Journal of Radiation Applications and Instrumentation. Part A. Applied Radiation and Isotopes. 40 (2): 171–176. doi:10.1016/0883-2889(89)90194-9.
- ^ Ivanov, T. B.; Mondeshka, Diana M.; Angelova, Ivanka G. (1989). "Verbesserte Synthese von 8-Amino-2-methyl-4-phenyl-1,2,3,4-Tetrahydroisochinolin". Journal für Praktische Chemie. 331 (5): 731–735. doi:10.1002/prac.19893310505.
- ^ Venkov, Atanas P.; Vodenicharov, Daniel M. (1990). "A New Synthesis of 1,2,3,4-Tetrahydro-2-methyl-4-phenylisoquinolines". Synthesis. 1990 (03): 253–255. doi:10.1055/s-1990-26846.
- ^ Pechulis, Anthony D.; Beck, James P.; Curry, Matt A.; Wolf, Mark A.; Harms, Arthur E.; Xi, Ning; Opalka, Chet; Sweet, Mark P.; Yang, Zhicai; Vellekoop, A. Samuel; Klos, Andrew M.; Crocker, Peter J.; Hassler, Carla; Laws, Mia; Kitchen, Douglas B.; Smith, Mark A.; Olson, Richard E.; Liu, Shuang; Molino, Bruce F. (2012). "4-Phenyl tetrahydroisoquinolines as dual norepinephrine and dopamine reuptake inhibitors". Bioorganic & Medicinal Chemistry Letters. 22 (23): 7219–7222. doi:10.1016/j.bmcl.2012.09.050.
- ^ Kunstmann, Rudolf; Gerhards, Hermann; Kruse, Hansjoerg; Leven, Margret; Paulus, Erich F.; Schacht, Ulrich; Schmitt, Karl; Witte, Peter U. (1987). "Resolution, absolute stereochemistry, and enantioselective activity of nomifensine and hexahydro-1H-indeno[1,2-b]pyridines". Journal of Medicinal Chemistry. 30 (5): 798–804. doi:10.1021/jm00388a009.
- ^ Jellinger K, Koeppen D, Rössner M. Langzeitbehandlung depressiver Syndrome mit Psyton [Long-term treatment of depressive syndromes with Psyton (author's transl)]. Wien Med Wochenschr. 1982 Apr 30;132(8):183-8. German. PMID 6125057.
