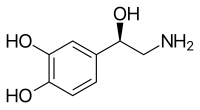
Norepinephrine
COMT | |
| Identifiers | |
|---|---|
| |
JSmol) | |
| |
| |
Norepinephrine (NE), also called noradrenaline (NA) or noradrenalin, is an
The general function of norepinephrine is to mobilize the brain and body for action. Norepinephrine release is lowest during sleep, rises during wakefulness, and reaches much higher levels during situations of stress or danger, in the so-called
In the brain, noradrenaline is produced in
A variety of medically important drugs work by altering the actions of noradrenaline systems.
Structure
Norepinephrine is a
Biochemical mechanisms
Biosynthesis
dopamine β-hydroxylase (DBH) enzyme . |
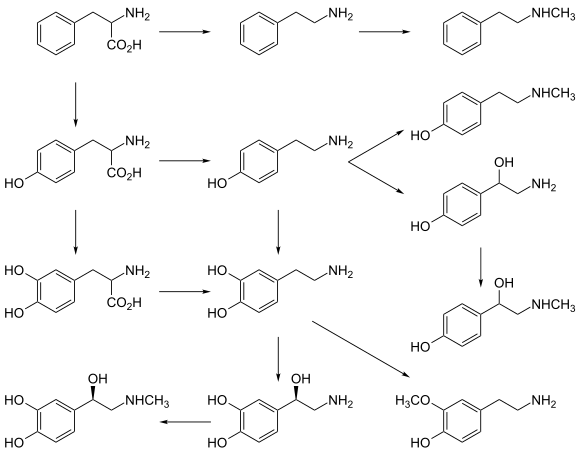
Norepinephrine is
- Phenylalanine → Tyrosine → L-DOPA → Dopamine → Norepinephrine[11]
Thus the direct precursor of norepinephrine is dopamine, which is synthesized indirectly from the essential amino acid phenylalanine or the non-essential amino acid tyrosine.[11] These amino acids are found in nearly every protein and, as such, are provided by ingestion of protein-containing food, with tyrosine being the most common.
Phenylalanine is converted into tyrosine by the enzyme
Norepinephrine itself can further be converted into
Degradation
In mammals, norepinephrine is rapidly degraded to various

Functions
Cellular effects
| Family | Receptor | Type | Mechanism |
|---|---|---|---|
Alpha
|
α1 | Gq-coupled. | Increase IP3 and calcium by activating phospholipase C. |
| α2 | Gi/Go-coupled. | Decrease adenylate cyclase .
| |
Beta
|
β1 | Gs-coupled. | Increase adenylate cyclase .
|
| β2 | |||
| β3 |
Like many other biologically active substances, norepinephrine exerts its effects by binding to and activating receptors located on the surface of cells. Two broad families of norepinephrine receptors have been identified, known as alpha and beta adrenergic receptors.[13] Alpha receptors are divided into subtypes α1 and α2; beta receptors into subtypes β1, β2, and β3.[13] All of these function as G protein-coupled receptors, meaning that they exert their effects via a complex second messenger system.[13] Alpha-2 receptors usually have inhibitory effects, but many are located pre-synaptically (i.e., on the surface of the cells that release norepinephrine), so the net effect of alpha-2 activation is often a decrease in the amount of norepinephrine released.[13] Alpha-1 receptors and all three types of beta receptors usually have excitatory effects.[13]
Storage, release, and reuptake

Inside the brain norepinephrine functions as a
Once in the synapse, norepinephrine binds to and activates receptors. After an action potential, the norepinephrine molecules quickly become unbound from their receptors. They are then absorbed back into the presynaptic cell, via reuptake mediated primarily by the norepinephrine transporter (NET).[16] Once back in the cytosol, norepinephrine can either be broken down by monoamine oxidase or repackaged into vesicles by VMAT, making it available for future release.[15]
Sympathetic nervous system

Norepinephrine is the main neurotransmitter used by the sympathetic nervous system, which consists of about two dozen
Broadly speaking, the effect of norepinephrine on each target organ is to modify its state in a way that makes it more conducive to active body movement, often at a cost of increased energy use and increased wear and tear.[18] This can be contrasted with the acetylcholine-mediated effects of the parasympathetic nervous system, which modifies most of the same organs into a state more conducive to rest, recovery, and digestion of food, and usually less costly in terms of energy expenditure.[18]
The sympathetic effects of norepinephrine include:
- In the eyes, an increase in production of tears, making the eyes more moist,iris dilator.
- In the heart, an increase in the amount of blood pumped.[20]
- In brown adipose tissue, an increase in calories burned to generate body heat (thermogenesis).[21]
- Multiple effects on the immune system. The sympathetic nervous system is the primary path of interaction between the immune system and the brain, and several components receive sympathetic inputs, including the thymus, spleen, and lymph nodes. However the effects are complex, with some immune processes activated while others are inhibited.[22]
- In the arteries, constriction of blood vessels, causing an increase in blood pressure.[23]
- In the
- In the liver, an increase in production of glucose, either by glycogenolysis after a meal or by gluconeogenesis when food has not recently been consumed.[24] Glucose is the body's main energy source in most conditions.
- In the pancreas, increased release of glucagon, a hormone whose main effect is to increase the production of glucose by the liver.[24]
- In skeletal muscles, an increase in glucose uptake.[24]
- In adipose tissue (i.e., fat cells), an increase in lipolysis, that is, conversion of fat to substances that can be used directly as energy sources by muscles and other tissues.[24]
- In the stomach and intestines, a reduction in digestive activity. This results from a generally inhibitory effect of norepinephrine on the enteric nervous system, causing decreases in gastrointestinal mobility, blood flow, and secretion of digestive substances.[25]
Noradrenaline and
Central nervous system

The noradrenergic neurons in the brain form a
The most important source of norepinephrine in the brain is the locus coeruleus, which contains noradrenergic cell group A6 and adjoins cell group A4. The locus coeruleus is quite small in absolute terms—in primates it is estimated to contain around 15,000 neurons, less than one-millionth of the neurons in the brain—but it sends projections to every major part of the brain and also to the spinal cord.[31]
The level of activity in the locus coeruleus correlates broadly with vigilance and speed of reaction. LC activity is low during sleep and drops to virtually nothing during the REM (dreaming) state.[32] It runs at a baseline level during wakefulness, but increases temporarily when a person is presented with any sort of stimulus that draws attention. Unpleasant stimuli such as pain, difficulty breathing, bladder distension, heat or cold generate larger increases. Extremely unpleasant states such as intense fear or intense pain are associated with very high levels of LC activity.[31]
Norepinephrine released by the locus coeruleus affects brain function in a number of ways. It enhances processing of sensory inputs, enhances attention, enhances formation and retrieval of both long term and working memory, and enhances the ability of the brain to respond to inputs by changing the activity pattern in the prefrontal cortex and other areas.[33] The control of arousal level is strong enough that drug-induced suppression of the LC has a powerful sedating effect.[32]
There is great similarity between situations that activate the locus coeruleus in the brain and situations that activate the sympathetic nervous system in the periphery: the LC essentially mobilizes the brain for action while the sympathetic system mobilizes the body. It has been argued that this similarity arises because both are to a large degree controlled by the same brain structures, particularly a part of the brainstem called the
Skin
Norepinephrine is also produced by Merkel cells which are part of the somatosensory system. It activates the afferent sensory neuron.[34]
Pharmacology
A large number of important drugs exert their effects by interacting with norepinephrine systems in the brain or body. Their uses include treatment of cardiovascular problems, shock, and a variety of psychiatric conditions. These drugs are divided into: sympathomimetic drugs which mimic or enhance at least some of the effects of norepinephrine released by the sympathetic nervous system; sympatholytic drugs, in contrast, block at least some of the effects.[35] Both of these are large groups with diverse uses, depending on exactly which effects are enhanced or blocked.[35]
Antagonists
Beta blockers
These are
However, the usefulness of beta blockers is limited by a range of serious side effects, including slowing of heart rate, a drop in blood pressure, asthma, and reactive hypoglycemia.[40] The negative effects can be particularly severe in people with diabetes.[37]
Alpha blockers
These are sympatholytic drugs that block the effects of adrenergic alpha receptors while having little or no effect on beta receptors.[43] Drugs belonging to this group can have very different effects, however, depending on whether they primarily block alpha-1 receptors, alpha-2 receptors, or both. Alpha-2 receptors, as described elsewhere in this article, are frequently located on norepinephrine-releasing neurons themselves and have inhibitory effects on them; consequently, blockage of alpha-2 receptors usually results in an increase in norepinephrine release.[43] Alpha-1 receptors are usually located on target cells and have excitatory effects on them; consequently, blockage of alpha-1 receptors usually results in blocking some of the effects of norepinephrine.[43] Drugs such as phentolamine that act on both types of receptors can produce a complex combination of both effects. In most cases when the term "alpha blocker" is used without qualification, it refers to a selective alpha-1 antagonist.
Selective
Some antidepressants function partly as selective
Alpha-2 agonists
These are
Stimulants and antidepressants
These are drugs whose primary effects are thought to be mediated by different neurotransmitter systems (dopamine for stimulants, serotonin for antidepressants), but many also increase levels of norepinephrine in the brain.[52] Amphetamine, for example, is a stimulant that increases release of norepinephrine as well as dopamine.[53] Monoamine oxidase A inhibitors (MAO-A) are antidepressants that inhibit the metabolic degradation of norepinephrine as well as serotonin and dopamine.[54] In some cases it is difficult to distinguish the norepinephrine-mediated effects from the effects related to other neurotransmitters.[citation needed]
Diseases and disorders
A number of important medical problems involve dysfunction of the norepinephrine system in the brain or body.
Sympathetic hyperactivation
Hyperactivation of the sympathetic nervous system is not a recognized condition in itself, but it is a component of a number of conditions, as well as a possible consequence of taking sympathomimetic drugs. It causes a distinctive set of symptoms including aches and pains, rapid heartbeat, elevated blood pressure, sweating, palpitations, anxiety, headache, paleness, and a drop in blood glucose. If sympathetic activity is elevated for an extended time, it can cause weight loss and other stress-related body changes.
The list of conditions that can cause sympathetic hyperactivation includes severe brain injury,[55] spinal cord damage,[56] heart failure,[57] high blood pressure,[58] kidney disease,[59] and various types of stress.
Pheochromocytoma
A pheochromocytoma is a rarely occurring tumor of the adrenal medulla, caused either by genetic factors or certain types of cancer. The consequence is a massive increase in the amount of norepinephrine and epinephrine released into the bloodstream. The most obvious symptoms are those of sympathetic hyperactivation, including particularly a rise in blood pressure that can reach fatal levels. The most effective treatment is surgical removal of the tumor.
Stress
ADHD
Attention deficit hyperactivity disorder is a neurodevelopmental condition involving problems with attention, hyperactivity, and impulsiveness.[61] It is most commonly treated using stimulant drugs such as methylphenidate (Ritalin), whose primary effect is to increase dopamine levels in the brain, but drugs in this group also generally increase brain levels of norepinephrine, and it has been difficult to determine whether these actions are involved in their clinical value. There is also substantial evidence that many people with ADHD show biomarkers involving altered norepinephrine processing.[62] Several drugs whose primary effects are on norepinephrine, including guanfacine, clonidine, and atomoxetine, have been tried as treatments for ADHD, and found to have effects comparable to those of stimulants.[63][64]
Autonomic failure
Several conditions, including Parkinson's disease, diabetes and so-called pure autonomic failure, can cause a loss of norepinephrine-secreting neurons in the sympathetic nervous system. The symptoms are widespread, the most serious being a reduction in heart rate and an extreme drop in resting blood pressure, making it impossible for severely affected people to stand for more than a few seconds without fainting. Treatment can involve dietary changes or drugs.[65]
REM sleep deprivation
Norepiprephine prevents REM sleep, and lack of REM sleep increases noradrenaline secretion[66] as a result of the locus coeruleus not ceasing producing it. It causes neurodegeneration if its loss is sustained for several days.[67]
Comparative biology and evolution
Norepinephrine has been reported to exist in a wide variety of animal species, including
History
Early in the twentieth century Walter Cannon, who had popularized the idea of a sympathoadrenal system preparing the body for fight and flight, and his colleague Arturo Rosenblueth developed a theory of two sympathins, sympathin E (excitatory) and sympathin I (inhibitory), responsible for these actions.[72] The Belgian pharmacologist Zénon Bacq as well as Canadian and U.S. pharmacologists between 1934 and 1938 suggested that noradrenaline might be a sympathetic transmitter.[72] In 1939, Hermann Blaschko and Peter Holtz independently identified the biosynthetic mechanism for norepinephrine in the vertebrate body.[73][74] In 1945 Ulf von Euler published the first of a series of papers that established the role of norepinephrine as a neurotransmitter.[75] He demonstrated the presence of norepinephrine in sympathetically innervated tissues and brain, and adduced evidence that it is the sympathin of Cannon and Rosenblueth.
Stanley Peart was the first to demonstrate the release of noradrenaline after the stimulation of sympathetic nerves.
References
- PMID 1202890.
- PMID 10678871.
- ^ "(−)-noradrenaline". IUPHAR database. International Union of Basic and Clinical Pharmacology. Retrieved 2 January 2016.
- ^ a b "Norepinephrine". PubChem. Retrieved 6 November 2015.
- S2CID 4284979.
- PMID 27512962.
- OCLC 1081403059.
- PMID 19948186.
- PMID 15860375.
- PMID 24374199.
- ^ ISBN 978-1-4684-3171-1.
- ISBN 978-1-60913-345-0.
- ^ ISBN 978-0-7020-5497-6.
- PMID 22717696.
- ^ S2CID 20764857.
- S2CID 21545649.
- ^ ISBN 978-0-12-386525-0.
- ^ ISBN 978-0-230-34367-2.
- PMID 19376264.
- PMID 25589262.
- PMID 26404650.
- PMID 24944034.
- S2CID 8150131.
- ^ PMID 26064978.
- PMID 15082874.
- PMID 17641668.
- PMID 14229500.
- S2CID 14046. Archived from the original(PDF) on 6 March 2019.
- PMID 20962208.
- PMID 22173709.
- ^ PMID 23040811.
- ^ PMID 22296742.
- S2CID 206952441.
- PMID 31001848.
- ^ ISBN 978-0-323-27714-3.
- S2CID 52827184. Archived from the original(PDF) on 1 March 2021.
We recommend norepinephrine as the first-choice vasopressor (strong recommendation, moderate quality of evidence).
- ^ S2CID 22137234.
- S2CID 3393256.
- PMID 25606393.
- ^ PMID 24872675.
- PMID 21987072.
- PMID 22783779.
- ^ ISBN 978-0-323-29361-7.
- PMID 25125424.
- PMID 29620795.
- S2CID 40069887.
- ^ PMID 25025070.
- .
- ^ PMID 15283516.
- PMID 26322115.
- PMID 3062194.
- PMID 18811678.
- PMID 23539642.
- PMID 27803666.
Selective inhibition of MAO-A leads to increased levels of neurotransmitter within noradrenergic (NA-ergic) and 5-HT-ergic neurons of the CNS, and clinical antidepressant action, while inhibition of MAO-B leads to increased levels of DA in the Parkinsonian brain...
- S2CID 10849388.
- S2CID 32173637.
- PMID 26413230.
- PMID 20393193.
- PMID 18089457.
- ^ S2CID 2259451.
- PMID 20815868.
- S2CID 36702503.
- PMID 26170637.
- S2CID 22649093.
- PMID 21664375.
- PMID 33240449.
- PMID 27014180.
- ^ .
- PMID 17101286.
- PMID 25696823.
- PMID 20621695.
- ^ ISBN 978-0-444-80493-8.
- ^ Blaschko H (1987). "A half-century of research on catecholamine biosynthesis". Journal of Applied Cardiology: 171–183.
- S2CID 260483975.
- S2CID 4100718.
External links
 Media related to Norepinephrine at Wikimedia Commons
Media related to Norepinephrine at Wikimedia Commons