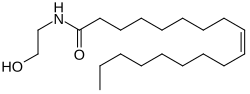
Oleoylethanolamide
| |
| Names | |
|---|---|
| Preferred IUPAC name
(9Z)-N-(2-Hydroxyethyl)octadec-9-enamide | |
| Identifiers | |
3D model (
JSmol ) |
|
| ChEBI | |
| ChemSpider | |
ECHA InfoCard
|
100.003.532 |
IUPHAR/BPS |
|
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C20H39NO2 | |
| Molar mass | 325.537 g·mol−1 |
| Appearance | White solid |
| Melting point | 59–60 °C (138–140 °F; 332–333 K) |
| Solubility in ethanol and DMSO | Soluble |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Oleoylethanolamide (OEA) is an
OEA is a shorter, monounsaturated
endocannabinoid anandamide, but unlike anandamide it acts independently of the cannabinoid pathway, regulating PPAR-α activity to stimulate lipolysis.[4]
OEA is produced by the
bile acids.[6]
OEA has been demonstrated to bind to the novel cannabinoid receptor GPR119.[7] OEA has been suggested to be the receptor's endogenous ligand.[8]
OEA has been hypothesized to play a key role in the inhibition of food seeking behavior and in the lipolysis of brown bears "
ursus arctos" during the hibernation season together with the alteration of the endocannabinoid system required for the metabolic changes for hibernation.[9]
OEA has been reported to lengthen the life span of the roundworm Caenorhabditis elegans through interactions with lysomal molecules.[10]
