Organometallic chemistry
Organometallic chemistry is the study of organometallic compounds,
Organometallic compounds are widely used both stoichiometrically in research and industrial chemical reactions, as well as in the role of catalysts to increase the rates of such reactions (e.g., as in uses of homogeneous catalysis), where target molecules include polymers, pharmaceuticals, and many other types of practical products.
Organometallic compounds

Organometallic compounds are distinguished by the prefix "organo-" (e.g., organopalladium compounds), and include all compounds which contain a bond between a metal atom and a carbon atom of an
A naturally occurring organometallic complex is
- Representative Organometallic Compounds
-
Ferrocene is an archetypal organoiron complex. It is an air-stable, sublimable compound.
-
Cobaltocene is a structural analogue of ferrocene, but is highly reactive toward air.
-
fragrances.
-
Zeise's salt is an example of a transition metal alkene complex.
-
Trimethylaluminium is an organometallic compound with a bridging methyl group. It is used in the industrial production of some alcohols.
-
Dimethylzinc has a linear coordination. It is a volatile pyrophoric liquid that is used in the preparation of semiconducting films.
-
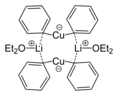
Lithium diphenylcuprate bis(diethyl etherate) is an example of a Gilman reagent, a type of organocopper complex frequently employed in organic synthesis.
-
Adenosylcobalamin is a cofactor required by several crucial enzymatic reactions that take place in the human body. It is a rare example of a metal (cobalt) alkyl in biology.
-
Iron(0) pentacarbonyl is a red-orange liquid prepared directly from the union of finely divided iron and carbon monoxide gas under pressure.
-
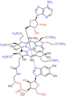
Technetium[99mTc] sestamibi is used to image the heart muscle in nuclear medicine.
Distinction from coordination compounds with organic ligands
Many
The status of compounds in which the
Structure and properties
The metal-carbon bond in organometallic compounds is generally highly

Most organometallic compounds are solids at room temperature, however some are liquids such as methylcyclopentadienyl manganese tricarbonyl, or even volatile liquids such as nickel tetracarbonyl.[1] Many organometallic compounds are air sensitive (reactive towards oxygen and moisture), and thus they must be handled under an inert atmosphere.[1] Some organometallic compounds such as triethylaluminium are pyrophoric and will ignite on contact with air.[6]
Concepts and techniques
As in other areas of chemistry,
A wide variety of physical techniques are used to determine the structure, composition, and properties of organometallic compounds.
Due to their high reactivity towards oxygen and moisture, organometallic compounds often must be handled using
History
Early developments in organometallic chemistry include
Recognition of organometallic chemistry as a distinct subfield culminated in the Nobel Prizes to Ernst Fischer and Geoffrey Wilkinson for work on metallocenes. In 2005, Yves Chauvin, Robert H. Grubbs and Richard R. Schrock shared the Nobel Prize for metal-catalyzed olefin metathesis.[13]
Organometallic chemistry timeline
- 1760 Louis Claude Cadet de Gassicourt isolates the organoarenic compound cacodyl
- 1827 olefincomplex
- 1848 Edward Frankland discovers diethylzinc
- 1890 nickel carbonyl
- 1899 John Ulric Nef discovers alkynylation using sodium acetylides.
- 1909 Salvarsanfor the treatment of syphilis, an early arsenic based organometallic compound
- 1912 Nobel Prize Victor Grignard and Paul Sabatier
- 1930 Henry Gilman invents lithium cuprates, see Gilman reagent
- 1940 organosiliconcompounds
- 1930's and 1940's Otto Roelen and Walter Reppe develop metal-catalyzed hydroformylation and acetylene chemistry
- 1951 Walter Hieber was awarded the Alfred Stock prize for his work with metal carbonyl chemistry.
- 1951 Ferrocene is discovered
- 1956 Dorothy Crawfoot Hodgkin determines the structure of vitamin B12, the first biomolecule found to contain a metal-carbon bond, see bioorganometallic chemistry
- 1963 Nobel prize for Karl Ziegler and Giulio Natta on Ziegler–Natta catalyst
- 1973 Nobel prize Geoffrey Wilkinson and Ernst Otto Fischer on sandwich compounds
- 1981 Nobel prize Roald Hoffmann and Kenichi Fukui for creation of the Woodward-Hoffman Rules
- 2001 R. Noyori and Karl Barry Sharplessfor asymmetric hydrogenation
- 2005 Richard Schrock on metal-catalyzed alkene metathesis
- 2010 Akira Suzukifor palladium catalyzed cross coupling reactions
Scope
Subspecialty areas of organometallic chemistry include:
- organoborane chemistry
- organosilicon chemistry
- organocalcium chemistry, organoscandium chemistry, organotitanium chemistry, organovanadium chemistry, organochromium chemistry, organomanganese chemistry, organoiron chemistry, organocobalt chemistry, organonickel chemistry, organocopper chemistry, organozinc chemistry, organogallium chemistry, organogermanium chemistry, organoarsenic chemistry, organoselenium chemistry
- organozirconium chemistry, organoniobium chemistry, organomolybdenum chemistry, organotechnetium chemistry, organoruthenium chemistry, organorhodium chemistry, organopalladium chemistry, organosilver chemistry, organocadmium chemistry, organoindium chemistry, organotin chemistry, organoantimony chemistry, organotellurium chemistry
- Period 7 elements: organoactinide chemistry, organothorium chemistry, organouranium chemistry, organoneptunium chemistry
Industrial applications
Organometallic compounds find wide use in commercial reactions, both as
Almost all processes involving carbon monoxide rely on catalysts, notable examples being described as

Almost all industrial processes involving
Most processes involving hydrogen rely on metal-based catalysts. Whereas bulk hydrogenations (e.g., margarine production) rely on heterogeneous catalysts, for the production of fine chemicals such hydrogenations rely on soluble (homogenous) organometallic complexes or involve organometallic intermediates.[18] Organometallic complexes allow these hydrogenations to be effected asymmetrically.
Many
Organometallic reactions
Organometallic compounds undergo several important reactions:
- associative and dissociative substitution
- oxidative addition and reductive elimination
- transmetalation
- migratory insertion
- β-hydride elimination
- electron transfer
- carbon-hydrogen bond activation
- carbometalation
- hydrometalation
- cyclometalation
- nucleophilic abstraction
The synthesis of many organic molecules are facilitated by organometallic complexes. Sigma-bond metathesis is a synthetic method for forming new carbon-carbon sigma bonds. Sigma-bond metathesis is typically used with early transition-metal complexes that are in their highest oxidation state.[19] Using transition-metals that are in their highest oxidation state prevents other reactions from occurring, such as oxidative addition. In addition to sigma-bond metathesis, olefin metathesis is used to synthesize various carbon-carbon pi bonds. Neither sigma-bond metathesis or olefin metathesis change the oxidation state of the metal.[20][21] Many other methods are used to form new carbon-carbon bonds, including beta-hydride elimination and insertion reactions.
Catalysis
Organometallic complexes are commonly used in catalysis. Major industrial processes include
Organometallic complexes are commonly used in small-scale fine chemical synthesis as well, especially in
Environmental concerns

Natural and contaminant organometallic compounds are found in the environment. Some that are remnants of human use, such as organolead and organomercury compounds, are toxicity hazards.
See also
References
- ^ a b c d e f g h i j Crabtree 2009, p. [page needed].
- ^
- ^ ISBN 978-3-527-29390-2.
- ^ Lippard & Berg 1994, p. [page needed].
- ISBN 978-0-12-809894-3.
- ^ "Triethylaluminium – SDS" (PDF). chemBlink. 24 May 2016. Retrieved 3 January 2021.
- ^ )
- ^ a b c d Shriver et al. 2014, p. [page needed].
- .
- CiteSeerX 10.1.1.693.9965.
- .
- ^ Crabtree 2009, p. 98.
- .
- ^ Elschenbroich 2016, p. [page needed].
- ISBN 978-3527306732.
- ^ a b Leeuwen 2005, p. [page needed].
- PMID 26151395.
- ISBN 978-3527306732.
- .
- ^ "Olefin Metathesis". The Organometallic HyperTextBook.
- ^ "Sigma Bond Metathesis". Organometallic HyperTextBook.
- PMID 21319862.
- PMID 25919276.
- PMID 21391570.
- .
- .
- PMID 27836476.
Sources
- Clayden, Jonathan; Greeves, Nick; Warren, Stuart (2012). Organic Chemistry. OUP Oxford. ISBN 978-0-19-927029-3.
- Crabtree, Robert H. (2009). The Organometallic Chemistry of the Transition Metals. John Wiley & Sons. ISBN 978-0-470-25762-3.
- Elschenbroich, Christoph (2016). Organometallics. John Wiley & Sons. ISBN 978-3-527-80514-3.
- Gupta, B. D; Elias, A J (2013). Basic Organometallic Chemistry: Concepts, Syntheses, and Applications of Transition Metals. Hyderabad: Universities Press. OCLC 903314566.
- Jenkins, Paul R. (1992). Organometallic Reagents in Synthesis. Oxford University Press. ISBN 978-0-19-855666-4.
- Leeuwen, Piet W. N. M. van (2005). Homogeneous Catalysis: Understanding the Art. Springer Science & Business Media. ISBN 978-1-4020-3176-2.
- Lippard, Stephen J.; Berg, Jeremy Mark (1994). Principles of Bioinorganic Chemistry. University Science Books. ISBN 978-0-935702-73-6.
- Pearson, Anthony J (1985). Metallo-organic chemistry. Wiley. OCLC 1200566627.
- Shriver, Duward; Weller, Mark; Overton, Tina; Armstrong, Fraser; Rourke, Jonathan (2014). Inorganic Chemistry. W. H. Freeman. ISBN 978-1-4292-9906-0.








![Technetium[99mTc] sestamibi is used to image the heart muscle in nuclear medicine.](http://upload.wikimedia.org/wikipedia/commons/thumb/1/17/Tc99_sestamibi_2D_structure.svg/100px-Tc99_sestamibi_2D_structure.svg.png)