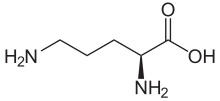
Ornithine
| |

| |
| Names | |
|---|---|
| IUPAC name
L-Ornithine
| |
| Other names
(+)-(S)-2,5-Diaminovaleric acid
(+)-(S)-2,5-Diaminopentanoic acid | |
| Identifiers | |
3D model (
JSmol ) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
ECHA InfoCard
|
100.000.665 |
| EC Number |
|
IUPHAR/BPS |
|
| KEGG | |
| MeSH | Ornithine |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties[1] | |
| C5H12N2O2 | |
| Molar mass | 132.16 g/mol |
| Melting point | 140 °C (284 °F; 413 K) |
| soluble | |
| Solubility | soluble in ethanol |
| Acidity (pKa) | 1.94 |
Chiral rotation ([α]D)
|
+11.5 (H2O, c = 6.5) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Ornithine is a
Role in urea cycle
L-Ornithine is one of the products of the action of the enzyme

Ornithine is not an amino acid coded for by DNA, that is, not proteinogenic. However, in mammalian non-hepatic tissues, the main use of the urea cycle is in arginine biosynthesis, so, as an intermediate in metabolic processes, ornithine is quite important.[4]
Other reactions
Ornithine, via the action of ornithine decarboxylase (E.C. 4.1.1.17), is the starting point for the synthesis of polyamines such as putrescine.
In bacteria, such as E. coli, ornithine can be synthesized from L-glutamate.[5]

Research
Exercise fatigue
L-Ornithine supplementation attenuated fatigue in subjects in a placebo-controlled study using a cycle ergometer. The results suggested that L-ornithine has an antifatigue effect in increasing the efficiency of energy consumption and promoting the excretion of ammonia.[6][7]
Weightlifting supplement
Cirrhosis
L-Ornithine L-aspartate (LOLA), a stable salt of ornithine and aspartic acid, has been used in the treatment of cirrhosis[10] and hepatic encephalopathy.[11]
References
- ISBN 0-8493-0462-8.
- PMID 27978498.
- PMID 10747936.
- S2CID 27957755.
- ^ "Ornithine Biosynthesis". School of Biological and Chemical Sciences, Queen Mary, University of London. Archived from the original on 2012-04-14. Retrieved 2007-08-17.
{{cite journal}}: Cite journal requires|journal=(help) - PMID 19083482.
- PMID 20717126.
- PMID 8220394.
- PMID 12093449.
- PMID 20642112.
- PMID 30706425.
