Osteoblast
| Osteoblast | |
|---|---|
bone tissue | |
| Identifiers | |
| Greek | osteoblastus |
| MeSH | D010006 |
| TH | H2.00.03.7.00002 |
| FMA | 66780 |
| Anatomical terms of microanatomy] | |
Osteoblasts (from the
Osteoblasts are specialized, terminally differentiated products of mesenchymal stem cells.[1] They synthesize dense, crosslinked collagen and specialized proteins in much smaller quantities, including osteocalcin and osteopontin, which compose the organic matrix of bone.
In organized groups of disconnected cells, osteoblasts produce
Bone structure
The
Bone remodeling
Bone is a dynamic tissue that is constantly being
Osteoblasts
Osteoblasts are the major cellular component of bone. Osteoblasts arise from
Normally, almost all of the bone matrix, in the air breathing
Osteoclasts
Osteoclasts are multinucleated cells that derive from hematopoietic progenitors in the bone marrow which also give rise to monocytes in peripheral blood.
Osteogenesis
Bone is formed by one of two processes:
During osteoblast
Bone morphogenetic proteins
Key growth factors in endochondral skeletal differentiation include bone morphogenetic proteins (BMPs) that determine to a major extent where chondrocyte differentiation occurs and where spaces are left between bones. The system of cartilage replacement by bone has a complex regulatory system. BMP2 also regulates early skeletal patterning. Transforming growth factor beta (TGF-β), is part of a superfamily of proteins that include BMPs, which possess common signaling elements in the TGF beta signaling pathway. TGF-β is particularly important in cartilage differentiation, which generally precedes bone formation for endochondral ossification. An additional family of essential regulatory factors is the fibroblast growth factors (FGFs) that determine where skeletal elements occur in relation to the skin
Steroid and protein hormones
Many other regulatory systems are involved in the transition of cartilage to bone and in bone maintenance. A particularly important bone-targeted hormonal regulator is
The skeleton is also modified for reproduction and in response to nutritional and other
Organization and ultrastructure
In well-preserved bone studied at high magnification via
Collagen and accessory proteins
Almost all of the organic (non-mineral) component of bone is dense collagen type I,[17] which forms dense crosslinked ropes that give bone its tensile strength. By mechanisms still unclear, osteoblasts secrete layers of oriented collagen, with the layers parallel to the long axis of the bone alternating with layers at right angles to the long axis of the bone every few micrometers. Defects in collagen type I cause the commonest inherited disorder of bone, called osteogenesis imperfecta.[18]
Minor, but important, amounts of small proteins, including osteocalcin and osteopontin, are secreted in bone's organic matrix.[19] Osteocalcin is not expressed at significant concentrations except in bone, and thus osteocalcin is a specific marker for bone matrix synthesis.[20] These proteins link organic and mineral component of bone matrix.[21] The proteins are necessary for maximal matrix strength due to their intermediate localization between mineral and collagen.
However, in mice where expression of osteocalcin or osteopontin was eliminated by targeted disruption of the respective genes (
Bone versus cartilage
The primitive skeleton is cartilage, a solid avascular (without blood vessels) tissue in which individual cartilage-matrix secreting cells, or chondrocytes, occur. Chondrocytes do not have intercellular connections and are not coordinated in units. Cartilage is composed of a network of collagen type II held in tension by water-absorbing proteins, hydrophilic proteoglycans.[24] This is the adult skeleton in cartilaginous fishes such as sharks. It develops as the initial skeleton in more advanced classes of animals.
In air-breathing vertebrates, cartilage is replaced by cellular bone. A transitional tissue is mineralized
Osteoblasts produce an advanced type of bone matrix consisting of dense, irregular crystals of hydroxyapatite, packed around the collagen ropes.[25] This is a strong composite material that allows the skeleton to be shaped mainly as hollow tubes. Reducing the long bones to tubes reduces weight while maintaining strength.
Mineralization of bone
The mechanisms of mineralization are not fully understood. Fluorescent, low-molecular weight compounds such as tetracycline or calcein bind strongly to bone mineral, when administered for short periods. They then accumulate in narrow bands in the new bone.[26] These bands run across the contiguous group of bone-forming osteoblasts. They occur at a narrow (sub-micrometer) mineralization front. Most bone surfaces express no new bone formation, no tetracycline uptake and no mineral formation. This strongly suggests that facilitated or active transport, coordinated across the bone-forming group, is involved in bone formation, and that only cell-mediated mineral formation occurs. That is, dietary calcium does not create mineral by mass action.
The mechanism of mineral formation in bone is clearly distinct from the
Osteoblasts separate bone from the extracellular fluid by tight junctions

At least one more regulated transport process is involved. The
- 6 HPO2−4 + 2 H2O + 10 Ca2+ ⇌ Ca10(PO4)6(OH)2 + 8 H+
In a closed system as mineral precipitates, acid accumulates, rapidly lowering the pH and stopping further precipitation. Cartilage presents no barrier to diffusion and acid therefore diffuses away, allowing precipitation to continue. In the osteon, where matrix is separated from extracellular fluid by tight junctions, this cannot occur. In the controlled, sealed compartment, removing H+ drives precipitation under a wide variety of extracellular conditions, as long as calcium and phosphate are available in the matrix compartment.[28] The mechanism by which acid transits the barrier layer remains uncertain. Osteoblasts have capacity for Na+/H+ exchange via the redundant Na/H exchangers, NHE1 and NHE6.[29] This H+ exchange is a major element in acid removal, although the mechanism by which H+ is transported from the matrix space into the barrier osteoblast is not known.
In bone removal, a reverse transport mechanism uses acid delivered to the mineralized matrix to drive hydroxyapatite into solution.[30]
Osteocyte feedback
Feedback from physical activity maintains bone mass, while feedback from osteocytes limits the size of the bone-forming unit.[31][32][33] An important additional mechanism is secretion by osteocytes, buried in the matrix, of sclerostin, a protein that inhibits a pathway that maintains osteoblast activity. Thus, when the osteon reaches a limiting size, it deactivates bone synthesis.[34]
Morphology and histological staining
-
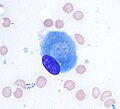
Osteoblast (Wright Giemsa stain, 100x)
-
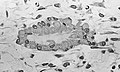
Light micrograph of decalcified cancellous bone displaying osteoblasts actively synthesizing osteoid, containing two osteocytes.
-
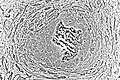
Light micrograph of undecalcified tissue displaying osteoblasts actively synthesizing osteoid (center).
-
Light micrograph of undecalcified tissue displaying osteoblasts actively synthesizing rudimentary bone tissue (center).
-
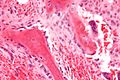
Osteoblasts lining bone (H&E stain).
Isolation of Osteoblasts
- The first isolation technique by microdissection method was originally described by Fell et al.[35] using chick limb bones which were separated into periosteum and remaining parts. She obtained cells which possessed osteogenic characteristics from cultured tissue using chick limb bones which were separated into periosteum and remaining parts. She obtained cells which possessed osteogenic characteristics from cultured tissue.
- Enzymatic digestion is one of the most advanced techniques for isolating bone cell populations and obtaining osteoblasts. Peck et al. (1964)[36] described the original method that is now often used by many researchers.
- In 1974 Jones et al.[37] found that osteoblasts moved laterally in vivo and in vitro under different experimental conditions and escribed the migration method in detail. The osteoblasts were, however, contaminated by cells migrating from the vascular openings, which might include endothelial cells and fibroblasts.
See also
- List of human cell types derived from the germ layers
- List of distinct cell types in the adult human body
References
- ^ PMID 10102814.
- PMID 14506899.
- ^ S2CID 20459725.
- S2CID 22605157.
- PMID 7047369.
- S2CID 43825140.
- ISBN 0-443-06583-7.
- PMID 18767962.
- S2CID 40654125.
- PMID 20421485.
- S2CID 7544706.
- PMID 18204096.
- PMID 24253048.
- ^ PMID 8527235.
- S2CID 29501339.
- S2CID 7632980.
- PMID 271986.
- S2CID 24461341.
- PMID 8579903.
- PMID 2939433.
- S2CID 20913443.
- PMID 9737340.
- PMID 20171304.
- ^ PMID 12023876.
- ^ PMID 21674636.
- S2CID 9373656.
- ^ ISBN 0-226-57512-8.[page needed]
- PMID 14498063.
- PMID 21413028.
- PMID 2528207.
- S2CID 9456704.
- . Retrieved 8 March 2022.
- PMID 32040934. Retrieved 8 March 2022.
- PMID 17118265.
- PMID 17104365.
- S2CID 26903706.
- S2CID 26078285.
Further reading
- ISBN 0-226-57512-8.
- ISBN 0-914168-88-6.