Redox
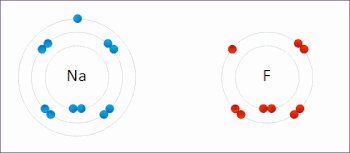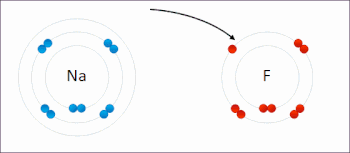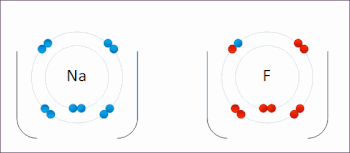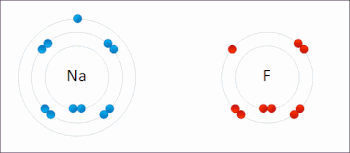
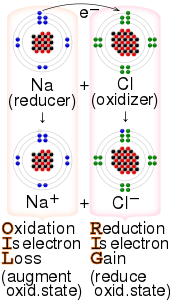
Redox ( or an increase in the oxidation state, while reduction is the gain of electrons or a decrease in the oxidation state.
There are two classes of redox reactions:
- Electron-transfer – Only one (usually) electron flows from the atom, ion or molecule being oxidized to the atom, ion, or molecule that is reduced. This type of redox reaction is often discussed in terms of redox couples and electrode potentials.
- rusting of iron, the oxidation state of iron atoms increases as the iron converts to an oxide, and simultaneously the oxidation state of oxygen decreases as it accepts electrons released by the iron. Although oxidation reactions are commonly associated with the formation of oxides, other chemical species can serve the same function.[5] In hydrogenation, bonds like C=C are reduced by transfer of hydrogen atoms.
Terminology
"Redox" is a
The processes of oxidation and reduction occur simultaneously and cannot occur independently.[5] In redox processes, the reductant transfers electrons to the oxidant. Thus, in the reaction, the reductant or reducing agent loses electrons and is oxidized, and the oxidant or oxidizing agent gains electrons and is reduced. The pair of an oxidizing and reducing agent that is involved in a particular reaction is called a redox pair. A redox couple is a reducing species and its corresponding oxidizing form,[7] e.g., Fe2+
/ Fe3+
.The oxidation alone and the reduction alone are each called a half-reaction because two half-reactions always occur together to form a whole reaction.[5]
Oxidants
Oxidation originally implied a reaction with oxygen to form an oxide. Later, the term was expanded to encompass
4), or else highly electronegative elements (e.g. O2, F2, Cl2, Br2, I2) that can gain extra electrons by oxidizing another substance.[3]
Oxidizers are oxidants, but the term is mainly reserved for sources of oxygen, particularly in the context of explosions. Nitric acid is a strong oxidizer.[9]
Reductants
Substances that have the ability to reduce other substances (cause them to gain electrons) are said to be reductive or reducing and are known as
Reductants in chemistry are very diverse.
Electronation and deelectronation
The
Rates, mechanisms, and energies
This section needs expansion. You can help by adding to it. (April 2023) |
Redox reactions can occur slowly, as in the formation of rust, or rapidly, as in the case of burning fuel. Electron transfer reactions are generally fast, occurring within the time of mixing.[citation needed]
The mechanisms of atom-transfer reactions are highly variable because many kinds of atoms can be transferred. Such reactions can also be quite complex, involving many steps. The mechanisms of electron-transfer reactions occur by two distinct pathways, inner sphere electron transfer and outer sphere electron transfer.[citation needed]
Analysis of bond energies and ionization energies in water allow calculation of the thermodynamic aspects of redox reactions.[citation needed]
Standard electrode potentials (reduction potentials)
This section needs additional citations for verification. (December 2023) |
Each half-reaction has a standard electrode potential (Eo
cell), which is equal to the potential difference or voltage at equilibrium under standard conditions of an electrochemical cell in which the cathode reaction is the half-reaction considered, and the anode is a standard hydrogen electrode where hydrogen is oxidized:
- 1⁄2H2 → H+ + e−
The electrode potential of each half-reaction is also known as its reduction potential (Eo
red), or potential when the half-reaction takes place at a cathode. The reduction potential is a measure of the tendency of the oxidizing agent to be reduced. Its value is zero for H+ + e− → 1⁄2H2 by definition, positive for oxidizing agents stronger than H+ (e.g., +2.866 V for F2) and negative for oxidizing agents that are weaker than H+ (e.g., −0.763V for Zn2+).[8]: 873
For a redox reaction that takes place in a cell, the potential difference is:
- Eo
cell = Eo
cathode – Eo
anode
However, the potential of the reaction at the anode is sometimes expressed as an oxidation potential:
- Eo
ox = –Eo
red
The oxidation potential is a measure of the tendency of the reducing agent to be oxidized but does not represent the physical potential at an electrode. With this notation, the cell voltage equation is written with a plus sign
- Eo
cell = Eo
red(cathode) + Eo
ox(anode)
Examples of redox reactions

In the reaction between hydrogen and fluorine, hydrogen is being oxidized and fluorine is being reduced:
- H2 + F2 → 2 HF
This reaction is spontaneous and releases 542 kJ per 2 g of hydrogen because the H-F bond is much stronger than the F-F bond. This reaction can be analyzed as two half-reactions. The oxidation reaction converts hydrogen to protons:
The reduction reaction converts fluorine to the fluoride anion:
- F2 + 2 e− → 2 F−
The half reactions are combined so that the electrons cancel:
H
2→ 2 H+ + 2 e− F
2 + 2 e−→ 2 F−
H2 + F2 → 2 H+ + 2 F−
The protons and fluoride combine to form hydrogen fluoride in a non-redox reaction:
- 2 H+ + 2 F− → 2 HF
The overall reaction is:
- H2 + F2 → 2 HF
Metal displacement

In this type of reaction, a metal atom in a compound or solution is replaced by an atom of another metal. For example, copper is deposited when zinc metal is placed in a copper(II) sulfate solution:
- Zn (s) + CuSO4 (aq) → ZnSO4 (aq) + Cu (s)
In the above reaction, zinc metal displaces the copper(II) ion from copper sulfate solution and thus liberates free copper metal. The reaction is spontaneous and releases 213 kJ per 65 g of zinc.
The ionic equation for this reaction is:
- Zn + Cu2+ → Zn2+ + Cu
As two half-reactions, it is seen that the zinc is oxidized:
- Zn → Zn2+ + 2 e−
And the copper is reduced:
- Cu2+ + 2 e− → Cu
Other examples
- The reduction of nitrate to nitrogen in the presence of an acid (denitrification):
- 2 NO−3 + 10 e− + 12 H+ → N2 + 6 H2O
- The combustion of hydrocarbons, such as in an internal combustion engine, produces water, carbon dioxide, some partially oxidized forms such as carbon monoxide, and heat energy. Complete oxidation of materials containing carbon produces carbon dioxide.
- The stepwise oxidation of a hydrocarbon by oxygen, in organic chemistry, produces water and, successively: an alcohol, an aldehyde or a ketone, a carboxylic acid, and then a peroxide.
Corrosion and rusting


- The term corrosion refers to the electrochemical oxidation of metals in reaction with an oxidant such as oxygen. Rusting, the formation of iron oxides, is a well-known example of electrochemical corrosion: it forms as a result of the oxidation of iron metal. Common rust often refers to iron(III) oxide, formed in the following chemical reaction:
- 4 Fe + 3 O2 → 2 Fe2O3
- The oxidation of iron(II) to iron(III) by hydrogen peroxide in the presence of an acid:
- Fe2+ → Fe3+ + e−
- H2O2 + 2 e− → 2 OH−
- Here the overall equation involves adding the reduction equation to twice the oxidation equation, so that the electrons cancel:
- 2 Fe2+ + H2O2 + 2 H+ → 2 Fe3+ + 2 H2O
Disproportionation
A disproportionation reaction is one in which a single substance is both oxidized and reduced. For example, thiosulfate ion with sulfur in oxidation state +2 can react in the presence of acid to form elemental sulfur (oxidation state 0) and sulfur dioxide (oxidation state +4).
- S2O2−3 + 2 H+ → S + SO2 + H2O
Thus one sulfur atom is reduced from +2 to 0, while the other is oxidized from +2 to +4.[8]: 176
Redox reactions in industry
Oxidation is used in a wide variety of industries such as in the production of cleaning products and oxidizing ammonia to produce nitric acid.[citation needed]
Redox reactions are the foundation of
Redox reactions in biology

Many important biological processes involve redox reactions. Before some of these processes can begin iron must be assimilated from the environment.[19]
Cellular respiration, for instance, is the oxidation of glucose (C6H12O6) to CO2 and the reduction of oxygen to water. The summary equation for cell respiration is:
- C6H12O6 + 6 O2 → 6 CO2 + 6 H2O + Energy
The process of cell respiration also depends heavily on the reduction of NAD+ to NADH and the reverse reaction (the oxidation of NADH to NAD+). Photosynthesis and cellular respiration are complementary, but photosynthesis is not the reverse of the redox reaction in cell respiration:
- 6 CO2 + 6 H2O + light energy → C6H12O6 + 6 O2
The term redox state is often used to describe the balance of
Redox cycling
Wide varieties of
Redox reactions in geology

Minerals are generally oxidized derivatives of metals. Iron is mined as its magnetite (Fe3O4). Titanium is mined as its dioxide, usually in the form of rutile (TiO2). To obtain the corresponding metals, these oxides must be reduced, which is often achieved by heating these oxides with carbon or carbon monoxide as reducing agents. Blast furnaces are the reactors where iron oxides and coke (a form of carbon) are combined to produce molten iron.The main chemical reaction producing the molten iron is:[20]
- Fe2O3 + 3 CO → 2 Fe + 3 CO2
Redox reactions in soils
Mnemonics
The key terms involved in redox can be confusing.
- "OIL RIG" — oxidation is loss of electrons, reduction is gain of electrons[24][25][26][27]
- "LEO the lion says GER [grr]" — loss of electrons is oxidation, gain of electrons is reduction[24][25][26][27]
- "LEORA says GEROA" — the loss of electrons is called oxidation (reducing agent); the gain of electrons is called reduction (oxidizing agent).[26]
- "RED CAT" and "AN OX", or "AnOx RedCat" ("an ox-red cat") — reduction occurs at the cathode and the anode is for oxidation
- "RED CAT gains what AN OX loses" – reduction at the cathode gains (electrons) what anode oxidation loses (electrons)
- "PANIC" – Positive Anode and Negative is Cathode. This applies to electrolytic cells which release stored electricity, and can be recharged with electricity. PANIC does not apply to cells that can be recharged with redox materials. These galvanic or voltaic cells, such as fuel cells, produce electricity from internal redox reactions. Here, the positive electrode is the cathode and the negative is the anode.
See also
- Anaerobic respiration
- Bessemer process
- Bioremediation
- Calvin cycle
- Chemical equation
- Chemical looping combustion
- Citric acid cycle
- Electrochemical series
- Electrochemistry
- Electrolysis
- Electron equivalent
- Electron transport chain
- Electrosynthesis
- Galvanic cell
- Hydrogenation
- Membrane potential
- Microbial fuel cell
- Murburn concept
- Nucleophilic abstraction
- Organic redox reaction
- Oxidative addition and reductive elimination
- Oxidative phosphorylation
- Partial oxidation
- Pro-oxidant
- Redox gradient
- Redox potential
- Reducing agent
- Reducing atmosphere
- Reduction potential
- Thermic reaction
- Transmetalation
- Sulfur cycle
References
- ^ "Metals". Bitesize. BBC. Archived from the original on November 3, 2022.
- ^ "redox – definition of redox in English | Oxford Dictionaries". Oxford Dictionaries | English. Archived from the original on October 1, 2017. Retrieved May 15, 2017.
- ^ ISBN 0-13-014329-4.
- ^ "Redox Reactions". wiley.com. Archived from the original on May 30, 2012. Retrieved May 9, 2012.
- ^ a b c Haustein, Catherine Hinga (2014). "Oxidation-reduction reaction". In K. Lee Lerner; Brenda Wilmoth Lerner (eds.). The Gale Encyclopedia of Science (5th ed.). Farmington Hills, MI: Gale Group.
- ^ Harper, Douglas. "redox". Online Etymology Dictionary.
- .
- ^ ISBN 978-0-13-293128-1.
- ^ "Nitric Acid Fact Sheet" (PDF). Department of Environmental Safety, Sustainability & Risk. University of Maryland. Retrieved February 12, 2024.
- ^ ISBN 0-03-072373-6.
- ISBN 81-219-2453-7.
- )
- ISBN 978-0-8412-3344-7.
- ISBN 978-0-8412-1780-5.
- ^ Bockris, John O'M.; Reddy, Amulya K. N. (1970). Modern Electrochemistry. Plenum Press. pp. 352–3.
- ISBN 9781461574675. Retrieved March 29, 2020.
The homogeneous proton-transfer reactions described are similar to homogeneous electron-transfer reactions in that the overall electron-transfer reaction can be decomposed into one electronation reaction and one deelectronation reaction.
- S2CID 242013948.
- ISBN 978-3527306732.
- ISBN 9780120007240. Retrieved September 10, 2023.
- ^ Bartlett, Richmond J.; James, Bruce R. (1991). "Redox chemistry of soils". Advances in Agronomy. 39: 151–208.
- ISBN 978-1-4398-0305-9.
- ^ ISBN 978-1-936137-74-9.
- ^ ISBN 978-0-02-828210-7.
- ^ ISBN 978-0-8400-6846-0.
- ^ ISBN 978-0-547-05405-6.
Further reading
- Schüring, J.; Schulz, H. D.; Fischer, W. R.; Böttcher, J.; Duijnisveld, W. H., eds. (1999). Redox: Fundamentals, Processes and Applications. Heidelberg: Springer-Verlag. p. 246. ISBN 978-3-540-66528-1.
- Tratnyek, Paul G.; Grundl, Timothy J.; Haderlein, Stefan B., eds. (2011). Aquatic Redox Chemistry. ACS Symposium Series. Vol. 1071. ISBN 978-0-8412-2652-4.