Pantothenate kinase-associated neurodegeneration
| Pantothenate kinase-associated neurodegeneration | |
|---|---|
| Other names | Neurodegeneration with brain iron accumulation 1 |
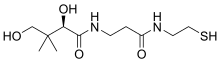 | |
| Pantetheine | |
| Specialty | Neurology |
| Symptoms | Dystonia, parkinsonism, dementia |
| Usual onset | Under 10 years (classical), Over 10 years (atypical) |
| Types | Classical, atypical |
| Causes | PANK2 mutation |
| Frequency | 1–3 per 1 million people |
Pantothenate kinase-associated neurodegeneration (PKAN), formerly called Hallervorden–Spatz syndrome,[1] is a genetic degenerative disease of the brain that can lead to parkinsonism, dystonia, dementia, and ultimately death. Neurodegeneration in PKAN is accompanied by an excess of iron that progressively builds up in the brain.
PKAN is caused by loss of function of the enzyme
Signs and symptoms
Symptoms typically begin in childhood and are progressive, often resulting in death by early adulthood. Symptoms of PKAN begin before middle childhood, and most often are noticed before ten years of age. Symptoms include:[citation needed]
- dystonia (repetitive uncontrollable muscle contractions that may cause jerking or twisting of certain muscle groups)
- dysphagia & dysarthria due to muscle groups involved in speech being involved
- rigidity/stiffness of limbs
- tremor
- writhing movements
- dementia
- spasticity
- weakness
- seizures (rare)
- toe walking
- night blindnessand later resulting in a complete loss of vision.
25% of individuals experience an uncharacteristic form of PKAN that develops post-10 years of age and follows a slower, more gradual pace of deterioration than those pre-10 years of age. These individuals face significant speech deficits as well as psychiatric and behavioral disturbances.[citation needed]
Being a progressive, degenerative nerve illness, PKAN leads to early immobility and often death by early adulthood. Death occurs prematurely due to infections such as pneumonia, and the disease in itself is technically not life limiting.[citation needed]
Genetics
PKAN is an
The disorder is caused by a mutant
PANK2 encodes a 1.85Kb transcript which is derived from seven exons covering a total distance of approximately 3.5Mb of genomic DNA. The PANK2 gene also encodes a 50.5-kDa
Mutant PANK2 gene coded proteins are often caused by null or missense mutations most notably a 7bp deletion in the PANK2 gene coding sequence.[citation needed]
This disorder has been reported in specific communities based on intra-community marriages where both parents of the child are carrying the same mutation. One of the communities reported is Agrawal (Agarwal) Community mainly based in Northern Part of India. The known mutation in Agarwal community is pathogenic mutation 1c.215_216insA in PANK2 gene. This is also coded as chr20:3870292-3870293insA by some labs. It results in a frameshift and premature truncation of the protein 47 amino acids downstream to codon 183 (p.Arg183GlufsTer47; ENST00000316562).[5][6]
Diagnosis

A neurological examination would show evidence of muscle rigidity; weakness; and abnormal postures, movements, and tremors. If other family members are also affected, this may help determine the diagnosis. Genetic tests can confirm an abnormal gene causing the disease. However, this test is not yet widely available. Other movement disorders and diseases must be ruled out. Individuals exhibiting any of the above listed symptoms are often tested using
Neuropathology
Microscopic features of PKAN include high levels of iron in the
Treatment
Phosphopantothenate has been shown to treat PKAN in a human, and also in a mouse model of the disease. Pantethine (a precursor of pantetheine) has been studied and shown to be effective in a mouse and in a fruit fly model of the disease.[9][10][11]
Prognosis
Survival rates for those diagnosed with typical PKAN, and left untreated is 11.18 years with a standard deviation of 7.8 years. A study reporting good outcomes in a single patient with late onset PKAN has been performed.[10]
Epidemiology
Prevalence data regarding this disorder remains incomplete, however it is estimated that anywhere between 1 in 1,000,000 to 3 in 1,000,000 individuals will be affected by this disorder (based upon observed cases in a population), but once again this is only an estimate as the disease is so rare it is difficult to statistically and accurately ascertain.[citation needed]
History
PKAN was first described by Hallervorden and Spatz (1922). Their discovery was brought about by a diagnosis of a family of 12 in which five sisters exhibited progressively increasing dementia and dysarthria. Autopsies revealed brown discolorations in different areas of the brain (particularly of interest were the globus pallidus and substantia nigra regions). Further investigation and description was brought about by Meyer (1958) who diagnosed 30 separate cases of PKAN. Meyer(1958) was followed by Elejalde et al. (1978) who described 5 affected family members and hypothesized that the disorder originated in central Europe, backing up his hypothesis with clinical and genetic analysis. Further investigation and insights were provided by Malmstrom-Groth and Kristensson (1982)[12] and Jankovic et al. (1985).[13]
Diagnosis of PKAN hit a milestone with the availability of MRIs, as well as the in-depth descriptions of those MRIs provided by Littrup and Gebarski (1985),[14] Tanfani et al. (1987),[15] Sethi et al. (1988),[16] Angelini et al. (1992),[17] Casteels et al. (1994),[18] and Malandrini et al. (1995).[19] The gene was localized to chromosome 20p by Taylor et al. (1996) [20] who suggested that this disorder should be referred to as neurodegeneration with brain iron accumulation (NBIA1) to avoid the objectionable eponym[21] of Hallervorden-Spatz. The disease was renamed 'pantothenate kinase-associated neurodegeneration' or PKAN by Zhou et al. (2001)[3] who suggested the name to avoid misinterpretation and to better reflect the true nature of the disorder. Most recently Pellecchia et al. (2005) published a report of 16 patients affected by PKAN, confirmed by genetic analysis.[22]
References
- S2CID 11594905.
- PMID 34909266.
- ^ S2CID 20400095.
- PMID 15747360.
- ^ "PANK2_Agarwal".
- ^ "Founder mutation in the PANK gene of Agrawal children with Neurodegeneration with Brain Iron accumulation (NBIA). :: Annals of Indian Academy of Neurology :: 2007 -- Britannica Online Encyclopedia". Archived from the original on 2011-02-12. Retrieved 2011-08-24.
- ^ a b Hanna, Philip A. "Pantothenate Kinase-Associated Neurodegeneration (PKAN)". Medscape. Retrieved 6 March 2020.
- National Library of Medicine. Retrieved 6 March 2020.
- PMID 24316510.
- ^ PMID 28567317.
- PMID 26549575.
- S2CID 35574844.
- PMID 3969211.
- PMID 3969211.
- PMID 3680689.
- S2CID 10181478.
- S2CID 11403203.
- S2CID 260241219.
- S2CID 37031359.
- S2CID 21893195.
- ^ Julius Hallervorden and Hugo Spatz were members of the Nazi party and had used executed political prisoners in medical research
- S2CID 23003382.
