Phendimetrazine
 | |
| Clinical data | |
|---|---|
| Trade names | Bontril |
| AHFS/Drugs.com | Monograph |
| Pregnancy category |
|
| Routes of administration | Oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | Peak plasma levels occur within 1 to 3 hours. Absorption is usually complete by 4 to 6 hours |
| Metabolism | Hepatic |
| Elimination half-life | 19-24 hours |
| Excretion | Urinary elimination |
| Identifiers | |
| |
JSmol) | |
| |
| |
| (verify) | |
Phendimetrazine (Bontril, Adipost, Anorex-SR, Appecon, Melfiat, Obezine, Phendiet, Plegine, Prelu-2, Statobex) is a
Pharmacology
Phendimetrazine functions as a
As an amphetamine congener, its structure incorporates the backbone of
Indicated as a short-term secondary treatment for exogenous obesity, phendimetrazine immediate-release 35mg tablets are typically consumed one hour before meals, not to exceed three doses daily. Phendimetrazine is also manufactured as a 105mg extended-release capsule for once daily dosing, typically consumed 30 to 60 minutes before a morning meal. Whereas the immediate-release formulation has a maximum daily dosage of 210mg (6 tablets), the extended-release capsules have a maximum daily dosage of 105mg (one capsule).
Legality
According to the List of Psychotropic Substances under International Control published by the International Narcotics Control Board, phendimetrazine is a Schedule III controlled substance under the Convention on Psychotropic Substances.[4]
Synthesis
Alkylation of ephedrine (1) with oxirane (2) gives the diol, N-(2-Hydroxyethyl) Pseudoephedrine [54275-43-3] [91688-17-4] (3). (The secondary nature of the amine in 1) eliminates the complication of dialkylation and thus the need to go through the amide.) Cyclization affords phendimetrazine (4).

The halogenation of Propiophenone [93-55-0] (1) with bromine gives 2-Bromopropiophenone [2114-00-3] (2). Further reaction with N-Methylethanolamine [109-83-1] (3) goes on to five CID:232614 [902267-47-4] (4). Cyclization with formic acid completed the synthesis of phendimetrazine (5).

The reaction between Ephedrine [299-42-3] (1) and ethyl chloroacetate [105-39-5] (2) in the presence of sodium hydride gives 4,5-dimethyl-6-phenylmorpholin-3-one, [5493-96-9] (3). The optional reduction of the lactam carbonyl with lithium aluminium hydride.
Additional method:[10]
See also
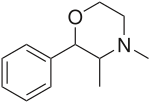
- 2-Phenyl-3,6-dimethylmorpholine
- 3-Fluorophenmetrazine
- Fenbutrazate
- G-130
- Morazone
- Manifaxine
- Radafaxine
- Phenmetrazine
- Fenmetramide
References
- ^ Anvisa (2023-03-31). "RDC Nº 784 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial" [Collegiate Board Resolution No. 784 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Diário Oficial da União (published 2023-04-04). Archived from the original on 2023-08-03. Retrieved 2023-08-16.
- PMID 18710555.
- PMID 17017961. Retrieved 2020-05-05.
- ^ "List of psychotropic substances under international control" (PDF). Archived from the original (PDF) on 2012-08-31. Retrieved 2005-06-15.
- ^ Otto, W. G. (7 March 1956). "Optisch aktives 2-Phenyl-3,4-dimethyl-morpholin". Angewandte Chemie. 68 (5): 181–182. doi:10.1002/ange.19560680508.
- ^ Werner Heel and Karl Zeile, U.S. patent 2,997,469 (1961 to Ingelheim, Germany, assignors to C. H. Boehringer Sohn, Ingelheim, Germany, a partnership).
- ^ , GB862198 (1961 to ILSE LIEBRECHT, JULIUS LIEBRECHT, WALTER MAYER LIST, WALTER MAYER-LIST).
- ^ , GB791416 (1958 to ILSE LIEBRECHT, JULIUS LIEBRECHT, WALTER MAYER LIST. WALTER MAYER-LIST).
- ^ Gannon Walter Francis & Poos George Ireland, U.S. patent 3,308,121 (1967 to Janssen Pharmaceuticals Inc).
- ^ https://eurekamag.com/research/031/845/031845550.php
