Phenyltropane

Phenyltropanes (PTs) were originally developed to reduce
Uses
Addiction
The phenyltropane compounds were initially discovered by R. Clarke et al. during research to try and dissociate the stimulant properties of cocaine from its abuse and dependence liability.[4][5] The first simple phenyltropanes to be made (WIN 35065-2 and WIN 34,428) were shown to be active in behavioral assays only for the ββ-isomers. The activity of the corresponding αβ-isomers was disappointing.
It was later shown that WIN 35065-2 and WIN 34,428 are mostly
Animal studies on monkeys and rats have tried to assess the self-administration propensity of phenyltropane analogs alongside cocaine. Frequently the analogs are administered prior to the start of a session to see if they can suppress cocaine lever responding. Most of the analogs behave in ways that might be considered typical for a DRI. In particular, they tend to stimulate locomotor activity, and cause nonselective reductions in cocaine intake relative to food.[10] At the dose that can reduce cocaine intake, most of the analogs require a high DAT occupancy.[11] This would mean that the agonists would need to be behaviorally active at the dose that can bring about reductions in cocaine craving. Most of the analogs will readily substitute for cocaine, although most do not elicit as many lever responses per session because of pharmacokinetic factors.[12] Since these agonists function as reinforcers, there is an obvious concern surrounding their abuse liability.
Nevertheless, a slow onset, long-duration agonist seems like a reasonable approach. Phenyltropanes are widely used in animal studies of
The more greatly attested habit creating methamphetamine is more serotonergic than the lesser reinforcing amphetamine. Most modern research suggests that 5-HT is negatively correlated with the addiction forming potential of psychostimulants, this is not saying that SRI properties cannot be considered beneficial. In fact, the above was proven by Rothman for releasing agents under the
Desipramine and atomoxetine are not reliably self-administered though, whereas most selective DRIs are. SSRIs are not self-administered either. Hence, it should be borne in mind that these neurotransmitters are unlikely to be involved in the addiction forming properties of cocaine and related stimulants. Nevertheless, they are still behaviorally active and will contribute to the effects that such drugs elicit in their users.
Promiscuity among transporters is worth bearing in mind. Monoamine transporters can transport neurotransmitters other than their "native" neurotransmitter.[15] As an example, in the prefrontal cortex where DATs are lower in number, DA is transported mostly by the NET instead. Hence, selective NRIs such as atomoxetine are able to increase the concentration of supracellular (synaptic) DA in this brain region via NET blockade.[16]
Weeding out SERT and NET affinity is desirable in the context that these molecular targets are less relevant to the goals of the treatment program, which is to reduce cocaine intake. It can be clearly seen that RTI-336 has fewer metabolically labile sites than cocaine, and therefore has a longer duration span.
Binding ligands
These compounds are primarily used in scientific research, as their high binding affinity for monoamine transporters, and the wide range of radiolabelled phenyltropane compounds available with different binding specificities makes them very useful for mapping the distribution of the various monoamine transporters in the brain.
Other uses
Some phenyltropane derivatives have also been researched for medical use in the treatment of conditions such as
Structure-activity relationships
Transporter selectivity
Compounds are known with a pronounced selectivity for each MAT – dopamine,[14] noradrenaline[18] and the serotonin transporter.[19]
Phenyltropane-based "
Isomers study
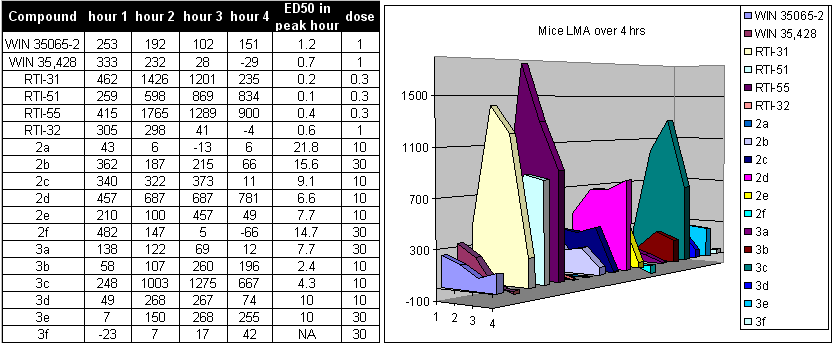
All of the tables and graphs shown beneath is from an article published by FIC, et al. 2004.[20] In summary the following observations can be made: Troparil, WIN35428 and RTI-32 are insufficiently potent. This observation is mainly based on the fact that at 100 mg/kg both troparil and WIN35428 produce convulsions. The twist-boat isomers are insufficiently potent in all cases. The trans isomers (alpha,beta) are too weak and might actually be dangerous and cause death. RTI-55, while highly potent, still causes death at a dose of 100 mg/kg. It is advised to consider RTI-229. RTI-31 is the most potent isomers for the DAT and was "safe" (on a relative scale) even in the event of overdose at 100 mg/kg. RTI-51 also looks like a "good" compound, although its synthesis is slightly more difficult than for RTI-31. RTI-51 is less selective for the DAT than RTI-31 and has appreciable SERT affinity also.

MAT binding affinities
| RTI | X | [3H]CFT | [3H]Nisoxetine | [3H]Paroxetine | N | S | N/D | S/D |
|---|---|---|---|---|---|---|---|---|
| — | H | 23 ± 5 | 920 ± 70 (550 ± 44) | 1960 ± 61 (178 ± 5.5) | 1.7 | 11 | 40 | 85.2 |
| — | F | 13.9 ± 2.0 | 835 ± 45 (503 ± 27) | 692 ± 27 (63 ± 2.5) | 1.7 | 11 | 60.1 | 49.8 |
| 31 | Cl | 1.1 ± 0.1 | 37 ± 2.1 (22 ± 1.3) | 44.5 ± 1.3 (4.0 ± 0.12) | 1.7 | 11 | 33.6 | 40.5 |
| 32 | Me | 1.7 ± 0.3 | 60 ± 0.53 (36 ± 0.32) | 240 ± 27 (23 ± 2.5) | 1.7 | 10 | 35.3 | 141 |
| 51 | Br | 1.7 ± 0.2 | 37.4 ± 5.2 (23 ± 3.1) | 10.6 ± 0.24 (0.96 ± 0.02) | 1.6 | 11 | 22 | 6.24 |
| 55 | I | 1.3 ± 0.01 | 36 ± 2.7 (22 ± 1.6) | 4.21 ± 0.30 (0.38 ± 0.03) | 1.6 | 11 | 27.7 | 3.24 |
| 2a | H | 101 ± 16 | 541 ± 69 (271 ± 34) | 5700 ± 720 (518 ± 66) | 2.0 | 11 | 5.36 | 56.4 |
| 2b | F | 21.0 ± 0.5 | 1200 ± 90 (741 ± 55) | 5060 ± 490 (460 ± 44) | 1.6 | 11 | 57.1 | 241 |
| 2c | Cl | 3.1 ± 0.6 | 5.14 ± 1.08 (3.1 ± 0.60) | 53 ± 3 (4.8 ± 0.26) | 1.7 | 11 | 1.66 | 17.1 |
| 2f | Me | 10.2 ± 0.8 | 270 ± 24 (160 ± 14) | 4250 ± 420 (390 ± 38) | 1.7 | 11 | 26.5 | 417 |
| 549 | Br | 1.7 ± 0.4 | 32.4 ± 3.5 (16.2 ± 1.7) | 84 ± 13.5 (20.6 ± 3.3) | 2.0 | 4.1 | 19.1 | 49.4 |
| 352 | I | 2.9 ± 0.2 | 52.4 ± 4.9 (32 ± 2.0) | 64.9 ± 1.97 (5.9 ± 0.18) | 1.6 | 11 | 18.1 | 22.4 |
| 3a | H | 670 ± 90 | >10000 | >10000 | — | |||
| 3b | F | 325 ± 8 | 7200 ± 810 (4340 ± 480) | >10000 | 1.7 | — | ||
| 3c | Cl | 25.0 ± 5 | 444 ± 29 (222 ± 15) | 1450 ± 160 (356 ± 40) | 2.0 | 4.1 | 17.8 | 58.0 |
| 3f | Me | 207 ± 21 | 2230 ± 380 (1120 ± 190) | >10000 | 2.0 | — | ||
| 3d | Br | 15.7 ± 0.9 | 272 ± 25 (136 ± 15) | 570 ± 80 (140 ± 20) | 2.0 | 4.1 | 17.3 | 36.3 |
| 3e | I | 22.7 ± 0.9 | 760 ± 49 (458 ± 30) | 66.3 ± 1.8 (6.0 ± 0.16) | 1.7 | 11 | 33.5 | 2.92 |
LMA, D.D. and G.B.
Related compounds
Closely related compounds have a varied
References
- ^ "The methyl has to be at the other O, and trans" does not put the methyl group somewhere else in the molecule: the ester group is oriented more outward leading to a less congestion around the methyl group. A second benefit to this orientation is freeing the nitrogen atom to form hydrogen bonding or even accept a proton to form a better soluble positive charged ion.
- ^ PMID 12723940.
- ^ PMID 17017960.
- ^ US patent 3813404, CLARKE R & DAUM S., "TROPANE-2-CARBOXYLATES AND DERIVATIVES", published 1974-05-28
- PMID 4747968.
- PMID 7910741.
- PMID 19128199.
- S2CID 25696981.
- PMID 11861788. Archived from the original(PDF) on 2006-09-21.
- PMID 18755212.
- S2CID 9205978.
- PMID 19086766.
- S2CID 70385136.
- ^ PMID 16584128.
- PMID 19022290.
- PMID 12431845.
- S2CID 7523758.
- PMID 15916437.
- PMID 8831768.
- PMID 15566309.
- PMID 15957006.
- PMID 17258302.