Physical organic chemistry
This article has multiple issues. Please help improve it or discuss these issues on the talk page. (Learn how and when to remove these template messages)
|
Physical organic chemistry, a term coined by
Application
Physical organic chemists use
Scope
Physical organic chemistry is the study of the relationship between structure and reactivity of
Physical organic chemists use both experimental and theoretical disciplines such as
History
The term physical organic chemistry was itself coined by
Chemical structure and thermodynamics
Thermochemistry
Organic chemists use the tools of
Empirical constants such as
The thermochemistry of reactive intermediates—
Conformational analysis
One of the primary methods for evaluating chemical stability and energetics is
In addition to molecular stability, conformational analysis is used to predict reaction products. One commonly cited example of the use of
The physical processes which give rise to
Non-covalent interactions
Chemists use the study of intramolecular and intermolecular
Acid–base chemistry
The properties of
—to predict relative acidities and basicities.The hard/soft acid/base principle is utilized to predict molecular interactions and reaction direction. In general, interactions between molecules of the same type are preferred. That is, hard acids will associate with hard bases, and soft acids with soft bases. The concept of hard acids and bases is often exploited in the synthesis of inorganic coordination complexes.
Kinetics
Physical organic chemists use the mathematical foundation of chemical kinetics to study the rates of reactions and reaction mechanisms. Unlike thermodynamics, which is concerned with the relative stabilities of the products and reactants (ΔG°) and their equilibrium concentrations, the study of kinetics focuses on the
Rate laws
The study of
Catalysis
The study of
Kinetic isotope effect
Although a rate law provides the stoichiometry of the
Substituent effects
The study of how substituents affect the reactivity of a molecule or the rate of reactions is of significant interest to chemists. Substituents can exert an effect through both
Other
Solvent effects
An example of the effect of solvent on organic reactions is seen in the
A modern example of the study of
Quantum chemistry
Many aspects of the structure-reactivity relationship in organic chemistry can be rationalized through
The energy associated with a particular
Due to complex interactions which arise from electron-electron repulsion, algebraic solutions of the Schrödinger equation are only possible for systems with one electron such as the
A complete electronic structure offers great predictive power for organic transformations and dynamics, especially in cases concerning
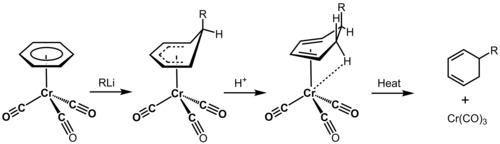
Quantum chemistry can also provide insight into the mechanism of an organic transformation without the collection of any experimental data. Because wavefunctions provide the total energy of a given molecular state, guessed molecular geometries can be optimized to give relaxed molecular structures very similar to those found through experimental methods.[20][page needed] Reaction coordinates can then be simulated, and transition state structures solved. Solving a complete energy surface for a given reaction is therefore possible, and such calculations have been applied to many problems in organic chemistry where kinetic data is unavailable or difficult to acquire.[1][page needed]
Spectroscopy, spectrometry, and crystallography
Physical organic chemistry often entails the identification of molecular structure, dynamics, and the concentration of reactants in the course of a reaction. The interaction of molecules with light can afford a wealth of data about such properties through nondestructive spectroscopic experiments, with light absorbed when the energy of a photon matches the difference in energy between two states in a molecule and emitted when an excited state in a molecule collapses to a lower energy state. Spectroscopic techniques are broadly classified by the type of excitation being probed, such as vibrational, rotational, electronic, nuclear magnetic resonance (NMR), and electron paramagnetic resonance spectroscopy. In addition to spectroscopic data, structure determination is often aided by complementary data collected from X-Ray diffraction and mass spectrometric experiments.[21][page needed]
NMR and EPR spectroscopy

One of the most powerful tools in physical organic chemistry is
Vibrational spectroscopy
Electronic excitation spectroscopy
Mass spectrometry
Crystallography

Unlike spectroscopic methods,
See also
- Journal of Physical Organic Chemistry
- Gaussian, an example of a commercially available quantum mechanical software package used. particularly, in academic settings
References
- ^ ISBN 9781891389313.[page needed]
- ISSN 0066-426X.
- .
- .
- ISBN 9780073047874.[page needed]
- ^ ISBN 978-0582218635.[page needed]
- S2CID 16332261.
- PMID 23327680.
- PMID 18563887.
- doi:10.1002/poc.2939.
- PMID 23851396.
- ^ ISBN 9780935702996. Retrieved 21 June 2015. Note, Amazon rather than Google allows access into this text.[page needed]
- .
- ISBN 978-3-527-32473-6.
- .
- .
- S2CID 6003410.
- ^ PMID 23978216.
- .
- ]
- ^ ]
- ^ James Keeler. "NMR and energy levels (Ch.2)" (PDF). Understanding NMR Spectroscopy. University of California, Irvine. Retrieved 2013-10-26.
- ISBN 978-0-470-74608-0.
- ^ Reusch, William. "Visible and Ultraviolet Spectroscopy". Michigan State University Website. Michigan State University. Retrieved 26 October 2013.
- ISBN 978-0-8493-0514-6.)
{{cite book}}:|first=has generic name (help)CS1 maint: multiple names: authors list (link - S2CID 3987932.
- S2CID 4105837.
Further reading
General
- Peter Atkins & Julio de Paula, 2006, "Physical chemistry," 8th Edn., New York, NY, USA:Macmillan, 1,3-butadieneby the Hückel method.]
- Thomas H. Lowry & Kathleen Schueller Richardson, 1987, Mechanism and Theory in Organic Chemistry, 3rd Edn., New York, NY, USA:Harper & Row, ISBN 0060440848, accessed 20 June 2015. [The authoritative textbook on the subject, containing a number of appendices that provide technical details on molecular orbital theory, kinetic isotope effects, transition state theory, and radical chemistry.]
- Eric V. Anslyn & Dennis A. Dougherty, 2006, Modern Physical Organic Chemistry, Sausalito, Calif.: University Science Books, ISBN 1891389319. [A modernized and streamlined treatment with an emphasis on applications and cross-disciplinary connections.]
- Michael B. Smith & Jerry March, 2007, "March's Advanced Organic Chemistry: Reactions, Mechanisms, and Structure," 6th Ed., New York, NY, USA:Wiley & Sons, ISBN 0470084944, accessed 19 June 2015.
- Francis A. Carey & Richard J. Sundberg, 2006, "Advanced Organic Chemistry: Part A: Structure and Mechanisms," 4th Edn., New York, NY, USA:Springer Science & Business Media, ISBN 0306468565, accessed 19 June 2015.
- Hammett, Louis P. (1940) Physical Organic Chemistry, New York, NY, USA: McGraw Hill, accessed 20 June 2015.
History
- Hammond, George S. (1997). "Physical organic chemistry after 50 years: It has changed, but is it still there?" (PDF). S2CID 53723796. Retrieved 20 June 2015. [An outstanding starting point on the history of the field, from a critically important contributor, referencing and discussing the early Hammett text, etc.]
Thermochemistry
- ISBN 0198025696, accessed 22 June 2015. (This book chapter surveys a very wide range of physical properties and their estimation, including the narrow list of thermochemical properties appearing in the June 2015 WP article, placing the Benson et al. method alongside many other methods. L. K. Doraiswamy is Anson Marston Distinguished Professor of Engineering at Iowa State University.)
- Irikura, Karl K.; Frurip, David J. (1998). "Computational Thermochemistry". In Irikura, Karl K.; Frurip, David J. (eds.). Computational Thermochemistry: Prediction and Estimation of Molecular Thermodynamics. ACS Symposium Series. Vol. 677. American Chemical Society. pp. 2–18. ISBN 978-0-8412-3533-5.

