Platinum
 | ||||||||||||||||||||||||||||||||||||||||||||||
| Platinum | ||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pronunciation | /ˈplætənəm/ | |||||||||||||||||||||||||||||||||||||||||||||
| Appearance | silvery white | |||||||||||||||||||||||||||||||||||||||||||||
| Standard atomic weight Ar°(Pt) | ||||||||||||||||||||||||||||||||||||||||||||||
| Platinum in the periodic table | ||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||
kJ/mol | ||||||||||||||||||||||||||||||||||||||||||||||
| Heat of vaporization | 510 kJ/mol | |||||||||||||||||||||||||||||||||||||||||||||
| Molar heat capacity | 25.86 J/(mol·K) | |||||||||||||||||||||||||||||||||||||||||||||
Vapor pressure
| ||||||||||||||||||||||||||||||||||||||||||||||
| Atomic properties | ||||||||||||||||||||||||||||||||||||||||||||||
Discovery | Antonio de Ulloa (1735) | |||||||||||||||||||||||||||||||||||||||||||||
| Isotopes of platinum | ||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||
Platinum is a
Platinum is a member of the
Platinum is one of the
Platinum is used in
Pure platinum is currently less expensive than pure
Characteristics
Physical
Pure platinum is a lustrous,
Its physical characteristics and chemical stability make it useful for industrial applications.[17] Its resistance to wear and tarnish is well suited to use in fine jewellery.
Chemical

Platinum has excellent resistance to
The most common
- Pt + 4 HNO3 + 6 HCl → H2PtCl6 + 4 NO2 + 4 H2O
As a soft acid, the Pt2+ ion has a great affinity for sulfide and sulfur ligands. Numerous DMSO complexes have been reported and care is taken in the choosing of reaction solvents.[23]
In 2007, the German scientist Gerhard Ertl won the Nobel Prize in Chemistry for determining the detailed molecular mechanisms of the catalytic oxidation of carbon monoxide over platinum (catalytic converter).[24]
Isotopes
Platinum has six naturally occurring
Pt, comprising 33.83% of all platinum. It is the only stable isotope with a non-zero spin. The spin of 1/2 and other favourable magnetic properties of the nucleus are utilised in 195
Pt NMR. Due to its spin and large abundance, 195
Pt satellite peaks are also often observed in 1
H and 31
P NMR spectroscopy (e.g., for Pt-phosphine and Pt-alkyl complexes). 190
Pt is the least abundant at only 0.01%. Of the naturally occurring isotopes, only 190
Pt is unstable, though it decays with a half-life of 6.5×1011 years, causing an activity of 15 Bq/kg of natural platinum. Other isotopes can undergo alpha decay, but their decay has never been observed, therefore they are considered stable.[25] Platinum also has 38 synthetic isotopes ranging in atomic mass from 165 to 208, making the total number of known isotopes 44. The least stable of these are 165
Pt and 166
Pt, with half-lives of 260 µs, whereas the most stable is 193
Pt with a half-life of 50 years. Most platinum isotopes decay by some combination of beta decay and alpha decay. 188
Pt, 191
Pt, and 193
Pt decay primarily by electron capture. 190
Pt and 198
Pt are predicted to have energetically favorable double beta decay paths.[26]
Occurrence



Platinum is an extremely rare metal,
In
In 1865,
Large platinum deposits are present in the state of Tamil Nadu, India.[37]
Platinum exists in higher abundances on the Moon and in meteorites. Correspondingly, platinum is found in slightly higher abundances at sites of bolide impact on Earth that are associated with resulting post-impact volcanism, and can be mined economically; the Sudbury Basin is one such example.[38]
Compounds
Halides
Hexachloroplatinic acid mentioned above is probably the most important platinum compound, as it serves as the precursor for many other platinum compounds. By itself, it has various applications in photography, zinc etchings,
Treatment of hexachloroplatinic acid with an ammonium salt, such as ammonium chloride, gives ammonium hexachloroplatinate,[21] which is relatively insoluble in ammonium solutions. Heating this ammonium salt in the presence of hydrogen reduces it to elemental platinum. Potassium hexachloroplatinate is similarly insoluble, and hexachloroplatinic acid has been used in the determination of potassium ions by gravimetry.[40]
When hexachloroplatinic acid is heated, it decomposes through platinum(IV) chloride and platinum(II) chloride to elemental platinum, although the reactions do not occur stepwise:[41]
- (H3O)2PtCl6·nH2O ⇌ PtCl4 + 2 HCl + (n + 2) H2O
- PtCl4 ⇌ PtCl2 + Cl2
- PtCl2 ⇌ Pt + Cl2
All three reactions are reversible. Platinum(II) and platinum(IV) bromides are known as well. Platinum hexafluoride is a strong oxidizer capable of oxidizing oxygen.
Oxides
- 2 Pt2+ + Pt4+ + 4 O2− → Pt3O4
Other compounds
Unlike
Several barium platinides have been synthesized in which platinum exhibits negative oxidation states ranging from −1 to −2. These include BaPt, Ba
3Pt
2, and Ba
2Pt.[44] Caesium platinide, Cs
2Pt, a dark-red transparent crystalline compound[45] has been shown to contain Pt2−
anions.[46] Platinum also exhibits negative oxidation states at surfaces reduced electrochemically.[47] The negative oxidation states exhibited by platinum are unusual for metallic elements, and they are attributed to the relativistic stabilization of the 6s orbitals.[46]
It is predicted that even the cation PtO2+
4 in which platinum exists in the +10 oxidation state may be achievable.[48]
Cisplatin, or cis-diamminedichloroplatinum(II) is the first of a series of square planar platinum(II)-containing chemotherapy drugs.[49] Others include carboplatin and oxaliplatin. These compounds are capable of crosslinking DNA, and kill cells by similar pathways to alkylating chemotherapeutic agents.[50] (Side effects of cisplatin include nausea and vomiting, hair loss, tinnitus, hearing loss, and nephrotoxicity.)[51][52]
Pt nuclear magnetic resonance spectroscopy
-
The hexachloroplatinate ion
-
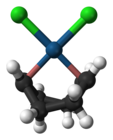
The anion of Zeise's salt
-
Dichloro(cycloocta-1,5-diene)platinum(II)
-
Cisplatin
History
Early uses
Archaeologists have discovered traces of platinum in the gold used in ancient Egyptian burials as early as 1200 BCE. For example, a small box from burial of Shepenupet II was found to be decorated with gold-platinum hieroglyphics.[53] However, the extent of early Egyptians' knowledge of the metal is unclear. It is quite possible they did not recognize there was platinum in their gold.[54][55]
The metal was used by Native Americans near modern-day Esmeraldas, Ecuador to produce artifacts of a white gold-platinum alloy. Archeologists usually associate the tradition of platinum-working in South America with the La Tolita Culture (c. 600 BCE – 200 CE), but precise dates and location are difficult, as most platinum artifacts from the area were bought secondhand through the antiquities trade rather than obtained by direct archeological excavation.[56] To work the metal, they would combine gold and platinum powders by sintering. The resulting gold–platinum alloy would then be soft enough to shape with tools.[57][58] The platinum used in such objects was not the pure element, but rather a naturally occurring mixture of the platinum group metals, with small amounts of palladium, rhodium, and iridium.[59]
European discovery
The first European reference to platinum appears in 1557 in the writings of the

In 1735, Antonio de Ulloa and Jorge Juan y Santacilia saw Native Americans mining platinum while the Spaniards were travelling through Colombia and Peru for eight years. Ulloa and Juan found mines with the whitish metal nuggets and took them home to Spain. Antonio de Ulloa returned to Spain and established the first mineralogy lab in Spain and was the first to systematically study platinum, which was in 1748. His historical account of the expedition included a description of platinum as being neither separable nor calcinable. Ulloa also anticipated the discovery of platinum mines. After publishing the report in 1748, Ulloa did not continue to investigate the new metal. In 1758, he was sent to superintend mercury mining operations in Huancavelica.[60]
In 1741, Charles Wood,[61] a British metallurgist, found various samples of Colombian platinum in Jamaica, which he sent to William Brownrigg for further investigation.
In 1750, after studying the platinum sent to him by Wood, Brownrigg presented a detailed account of the metal to the
Means of malleability
Because the other platinum-family members were not discovered yet (platinum was the first in the list), Scheffer and Sickingen made the false assumption that due to its hardness—which is slightly more than for pure iron—platinum would be a relatively non-pliable material, even brittle at times, when in fact its ductility and malleability are close to that of gold. Their assumptions could not be avoided because the platinum they experimented with was highly contaminated with minute amounts of platinum-family elements such as osmium and iridium, amongst others, which embrittled the platinum alloy. Alloying this impure platinum residue called "plyoxen"[citation needed] with gold was the only solution at the time to obtain a pliable compound, but nowadays, very pure platinum is available and extremely long wires can be drawn from pure platinum, very easily, due to its crystalline structure, which is similar to that of many soft metals.[64]
In 1786,
Production


Platinum, along with the rest of the
If pure platinum is found in
One suitable method for purification for the raw platinum, which contains platinum, gold, and the other platinum-group metals, is to process it with aqua regia, in which palladium, gold and platinum are dissolved, whereas osmium, iridium, ruthenium and rhodium stay unreacted. The gold is precipitated by the addition of iron(II) chloride and after filtering off the gold, the platinum is precipitated as ammonium chloroplatinate by the addition of ammonium chloride. Ammonium chloroplatinate can be converted to platinum by heating.[68] Unprecipitated hexachloroplatinate(IV) may be reduced with elemental zinc, and a similar method is suitable for small scale recovery of platinum from laboratory residues.[69] Mining and refining platinum has environmental impacts.[70]
Applications

Of the 218 tonnes of platinum sold in 2014, 98 tonnes were used for vehicle emissions control devices (45%), 74.7 tonnes for jewelry (34%), 20.0 tonnes for chemical production and petroleum refining (9.2%), and 5.85 tonnes for electrical applications such as hard disk drives (2.7%). The remaining 28.9 tonnes went to various other minor applications, such as medicine and biomedicine, glassmaking equipment, investment, electrodes, anticancer drugs, oxygen sensors, spark plugs and turbine engines.[71]
Catalyst
The most common use of platinum is as a
Green energy transition
As a fuel cell catalyst, platinum enables hydrogen and oxygen reactions to take place at an optimum rate. It is used in platinum-based proton exchange memebrane (PEM) technologies required in green hydrogen production as well as fuel cell electric vehicle adoption (FCEV).[75][76]
Standard

From 1889 to 1960, the
The Standard Platinum
The
As an investment
Platinum is a
In
During periods of sustained economic stability and growth, the price of platinum tends to be as much as twice the price of gold, whereas during periods of economic uncertainty,
-
1,000 cubic centimeters of 99.9% pure platinum, worth about US$696,000 at 29 Jun 2016 prices[88]
-
Platinum price 1970–2022
Other uses
In the laboratory, platinum wire is used for electrodes; platinum pans and supports are used in thermogravimetric analysis because of the stringent requirements of chemical inertness upon heating to high temperatures (~1000 °C). Platinum is used as an alloying agent for various metal products, including fine wires, noncorrosive laboratory containers, medical instruments, dental prostheses, electrical contacts, and thermocouples. Platinum-cobalt, an alloy of roughly three parts platinum and one part cobalt, is used to make relatively strong permanent magnets.[39] Platinum-based anodes are used in ships, pipelines, and steel piers.[22] Platinum drugs are used to treat a wide variety of cancers, including testicular and ovarian carcinomas, melanoma, small-cell and non-small-cell lung cancer, myelomas and lymphomas.[89]
Symbol of prestige in marketing
Platinum's rarity as a metal has caused advertisers to associate it with exclusivity and wealth. "Platinum"
Health problems
According to the Centers for Disease Control and Prevention, short-term exposure to platinum salts may cause irritation of the eyes, nose, and throat, and long-term exposure may cause both respiratory and skin allergies. The current OSHA standard is 2 micrograms per cubic meter of air averaged over an 8-hour work shift.[93] The National Institute for Occupational Safety and Health has set a recommended exposure limit (REL) for platinum as 1 mg/m3 over an 8-hour workday.[94]
As platinum is a
See also
References
- ^ "Standard Atomic Weights: Platinum". CIAAW. 2005.
- ISSN 1365-3075.
- ^ ISBN 978-1-62708-155-9.
- ISBN 0-8493-0464-4.
- .
- ^ "platinum (Pt)". Encyclopædia Britannica. Encyclopædia Britannica Inc. 2012. Archived from the original on 5 April 2012. Retrieved 24 April 2012.
- ^ Harper, Douglas. "platinum". Online Etymology Dictionary.
- ^ Hobson, Peter. "Currency shocks knock platinum to 10-year lows". Reuters. Retrieved 20 August 2018.
- ^ "Platinum in the Glass Industry". Johnson Matthey Technology Review.
- ^ "Chapter 6.11 Platinum" (PDF), Air Quality Guidelines (2nd ed.), WHO Regional Office for Europe, Copenhagen, Denmark, 2000, archived (PDF) from the original on 18 October 2012
- PMID 20593091.
- ^ "Platinum Prices vs Gold Prices".
- ^ "Live latinum Price Charts & Historical Data". APMEX. Retrieved 14 March 2021.
- ^ ISBN 978-0-02-865724-0.
- ISBN 9781420017168.
- ISBN 9780071360760.
- ISBN 978-0-87170-518-1. Archivedfrom the original on 24 March 2017.
- ^ Chaston, J.C. "Reaction of Oxygen with the Platinum Metals". technology.matthey.com. Retrieved 30 July 2022.
- . Retrieved 30 July 2022.
- ^ Sir Norman Lockyer (1891). Nature. Macmillan Journals Limited. pp. 625–. Archived from the original on 24 March 2017.
- ^ ISBN 978-0-470-13240-1.
- ^ ISBN 978-0-8493-0488-0.
- ^ .
- S2CID 38416086.
- S2CID 201664098.
- .
- .
- ISBN 978-92-2-109816-4. Archivedfrom the original on 24 March 2017.
- ^ Murata, K. J. (1958). in Symposium on Spectrocemical Analysis for Trace Elements. ASTM International. p. 71. Archived from the original on 24 March 2017.
- ^ "The History of Platinum". Alaska Community Database Online. ExploreNorth. Archived from the original on 22 December 2010. Retrieved 12 April 2011.
Platinum is located on the Bering Sea coast, below Red Mountain on the south spit of Goodnews Bay.
- .
- ^ Dan Oancea Platinum In South Africa Archived 13 August 2011 at the Wayback Machine. MINING.com. September 2008
- ^ R. Grant Cawthorn (1999). "Seventy-fifth Anniversary of the Discovery of the Platiniferous Merensky Reef". Platinum Metals Review. Retrieved 24 December 2017.
- ^ ISBN 978-0471238966.
- ^ "Mining Platinum in Montana". New York Times. 13 August 1998. Archived from the original on 3 February 2008. Retrieved 9 September 2008.
- ^ Loferski, P. J. (July 2012). "Platinum–Group Metals" (PDF). USGS Mineral Resources Program. Archived (PDF) from the original on 7 July 2012. Retrieved 17 July 2012.
- ^ "Evidence of huge deposits of platinum in State". The Hindu. Chennai, India. 2 July 2010. Archived from the original on 6 December 2011.
- ISBN 978-1-86239-017-1. Archivedfrom the original on 24 March 2017.
- ^ ISBN 978-0-313-30123-0.
- .
- .
- S2CID 4184615.
- .
- PMID 16479284.
- PMID 14562358.
- ^ .
- .
- PMID 27273799.
- PMID 29394020.
- PMID 17325786.
- ISBN 978-1-62652-816-1. Archivedfrom the original on 9 November 2017. Retrieved 11 June 2016.
- S2CID 24509477.
- ^ Berthelot, M. (1901). "Sur les métaux égyptiens: Présence du platine parmi les caractères d'inscriptions hiéroglyphiques, confié à mon examn" [On Egyptian metals: Presence of platinum among the characters of hieroglyphic inscriptions, entrusted to my examination]. Comptes rendus de l'Académie des Sciences (in French). 132: 729.
- ISBN 978-0-313-33507-5.
- S2CID 192364303.
- ^ David A. Scott and Warwick Bray (1980). "Ancient Platinum Technology in South America: Its use by the Indians in Pre-Hispanic Times". Platinum Metals Review. Retrieved 5 November 2018.
- S2CID 4100269.
- ISSN 0003-813X.
- ^ ISBN 978-0-905118-83-3.
- ^ OCLC 23991202.
- ^ Dixon, Joshua; Brownrigg, William (1801). The literary life of William Brownrigg. To which are added an account of the coal mines near Whitehaven: And Observations on the means of preventing epidemic fevers. p. 52. Archived from the original on 24 March 2017.
- S2CID 186213277.
- ^ Marggraf, Andreas Sigismund (1760). Versuche mit dem neuen mineralischen Körper Platina del pinto genannt. Archived from the original on 24 March 2017.
- ^ Platinum Archived 22 December 2011 at the Wayback Machine. mysite.du.edu
- ^ Kelly, Thomas D. and Matos, Grecia R. (2013)Historical Statistics for Mineral and Material Commodities in the United States Archived 4 June 2013 at the Wayback Machine, U.S. Geological Survey
- ^ Loferski, P. J. (October 2011). "2010 Minerals Yearbook; Platinum-group metals" (PDF). USGS Mineral Resources Program. Archived (PDF) from the original on 8 July 2012. Retrieved 17 July 2012.
- ISBN 978-0-8306-3018-9.
- (PDF) from the original on 29 October 2008.
- ISBN 978-0-470-13238-8.
- ^ Cairncross, E. (March 2014). "Health and environmental impacts of platinum mining: Report from South Africa" (PDF). Archived (PDF) from the original on 5 October 2016. Retrieved 4 October 2016.
- ^ Loferski, P. J. (July 2016). "2014 Minerals Yearbook; Platinum-group metals" (PDF). USGS Mineral Resources Program. Archived (PDF) from the original on 18 August 2016. Retrieved 11 July 2016.
- ISBN 978-0-13-149330-8.
- ISBN 978-0-470-84857-9.
- PMID 18399516.
- ^ Sterck, Edward (17 November 2023), "Why Platinum is a Strategically Important Metal", Singapore Bullion Market Association
- PMID 31479264.
- S2CID 150519250.
- International Committee for Weights and Measures. Archived from the original(PDF) on 24 February 2021. Retrieved 23 October 2020.
- NIST.
- .
- ^ "Professional Jeweler's Magazine Archives, issue of August 2004". Archived from the original on 28 September 2011. Retrieved 19 June 2011.
- ^ "Platinum primer". Diamond Cutters International. 12 December 2008. Archived from the original on 27 September 2011. Retrieved 18 June 2011.
- ^ "Unknown Facts about Platinum". watches.infoniac.com. Archived from the original on 21 September 2008. Retrieved 9 September 2008.
- ^ "Platinum versus Gold". The Speculative Invertor. 14 April 2002. Archived from the original on 26 October 2008.
- PMID 8575456.
- .
- ^ "Platinum". Minerals Zone. Archived from the original on 12 October 2008. Retrieved 9 September 2008.
- ^ "21.09kg Pt". WolframAlpha. Archived from the original on 23 August 2014. Retrieved 14 July 2012.
- PMID 26113607.
- .
- ISBN 978-0-88882-219-2. Archivedfrom the original on 24 March 2017.
- ISBN 978-1-4027-7004-3. Archivedfrom the original on 24 March 2017.
- ^ "Occupational Health Guideline for Soluble Platinum Salts (as Platinum)" (PDF). Centers for Disease Control and Prevention. Archived (PDF) from the original on 11 March 2010. Retrieved 9 September 2008.
- ^ "CDC – NIOSH Pocket Guide to Chemical Hazards – Platinum". www.cdc.gov. Archived from the original on 21 November 2015. Retrieved 21 November 2015.
- ^ "FDA Backgrounder on Platinum in Silicone Breast Implants". U.S. Food and Drug Administration. Archived from the original on 24 July 2008. Retrieved 9 September 2008.
- PMID 16483647.
- ^ "187 Fake Cancer 'Cures' Consumers Should Avoid". U.S. Food and Drug Administration. Archived from the original on 2 May 2017. Retrieved 20 May 2020.
Further reading
- Young, Gordon (November 1983). "The Miracle Metal—Platinum". OCLC 643483454.
External links
- Platinum at The Periodic Table of Videos(University of Nottingham)
- NIOSH Pocket Guide to Chemical Hazards – Platinum Centers for Disease Control and Prevention
- "The PGM Database". Archived from the original on 1 July 2019. Retrieved 5 November 2011.
- "A balanced historical account of the sequence of discoveries of platinum; illustrated".
- "Platinum-Group Metals Statistics and Information". United States Geological Survey, National Minerals Information Center.
- "International Platinum Group Metals Association".

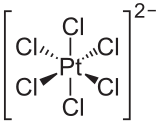


![1,000 cubic centimeters of 99.9% pure platinum, worth about US$696,000 at 29 Jun 2016 prices[88]](http://upload.wikimedia.org/wikipedia/commons/thumb/4/48/One_litre_of_Platinum.jpg/200px-One_litre_of_Platinum.jpg)
