Plerixafor
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Mozobil |
| Other names | JM 3100, AMD3100 |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a609018 |
| License data |
|
| Pregnancy category |
|
Subcutaneous | |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Protein binding | Up to 58% |
| Metabolism | None |
| Elimination half-life | 3–5 hours |
| Excretion | Kidney |
| Identifiers | |
| |
JSmol) | |
| |
| |
| | |
Plerixafor, sold under the brand name Mozobil, is an
Medical uses
Phase 2 clinical trials started in 2021 exploring the combination of plerixafor and MGTA-145, a CXCL2 ligand.[6][7]
Contraindications
Pregnancy and lactation
Studies in pregnant animals have shown
Adverse effects
Nausea, diarrhea and local reactions were observed in over 10% of patients. Other problems with digestion and general symptoms like dizziness, headache, and muscular pain are also relatively common; they were found in more than 1% of patients. Allergies occur in less than 1% of cases. Most adverse effects in clinical trials were mild and transient.[5][8]
The European Medicines Agency has listed a number of safety concerns to be evaluated on a post-marketing basis, most notably the theoretical possibilities of spleen rupture and tumor cell mobilisation. The first concern has been raised because splenomegaly was observed in animal studies, and G-CSF can cause spleen rupture in rare cases. Mobilisation of tumor cells has occurred in patients with leukaemia treated with plerixafor.[9]
Interactions
No interaction studies have been conducted. The fact that plerixafor does not interact with the cytochrome system indicates a low potential for interactions with other drugs.[5]
Pharmacology
Mechanism of action
In the form of its zinc complex, plerixafor acts as an
Pharmacokinetics
Following
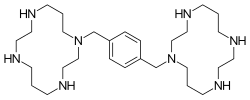
Chemistry
Plerixafor is a
Synthesis
Three of the four nitrogen atoms of the macrocycle cyclam... (1,4,8,11-tetraazacyclotetradecane) are protected with
History
The molecule was first synthesised in 1987 to carry out basic studies on the
Society and culture
Plerixafor has
Research
Anti-cancer properties
Plerixafor was seen to reduce metastasis in mice in several studies.[18] It has also been shown to reduce recurrence of glioblastoma in a mouse model after radiotherapy. In this model, the cancer cells that survived radiation critically depended on bone marrow derived cells for vasculogenesis, and the recruitment of the latter was mediated by SDF-1 CXCR4 interactions, which are blocked by plerixafor.[19]
Use in stem cell research
Researchers at Imperial College have demonstrated that plerixafor in combination with
In double‐blind, randomized, placebo‐controlled trial, stem cell mobilization with plerixafor did not improve healing of ischemic diabetic wounds.[21]
Neurologic
Blockade of CXCR4 signalling by plerixafor has also unexpectedly been found to be effective at counteracting opioid-induced hyperalgesia produced by chronic treatment with morphine, though only animal studies have been conducted as yet.[22]
References
- ^ "Product monograph brand safety updates". Health Canada. February 2024. Retrieved 24 March 2024.
- ^ "Mozobil- plerixafor injection, solution". DailyMed. 26 June 2023. Retrieved 13 September 2023.
- ^ "Plerixafor Accord EPAR". European Medicines Agency. 12 October 2022. Retrieved 7 February 2023.
- ^ S2CID 20824572.
- ^ ISBN 978-3-85200-196-8.
- ^ Stanford University (16 August 2021). "Phase II Study of MGTA-145 in Combination With Plerixafor in the Mobilization of Hematopoietic Stem Cells for Autologous Transplantation in Patients With Multiple Myeloma".
{{cite journal}}: Cite journal requires|journal=(help) - ^ Magenta Therapeutics, Inc. (23 August 2021). "A Phase II Study Evaluating the Safety and Efficacy of MGTA-145 in Combination With Plerixafor for the Mobilization and Transplantation of HLA-Matched Donor Hematopoietic Stem Cells in Recipients With Hematological Malignancies". National Marrow Donor Program.
{{cite journal}}: Cite journal requires|journal=(help) - S2CID 195684110.
- ^ a b "CHMP Assessment Report for Mozobil" (PDF). European Medicines Agency.
- S2CID 28540154.
- PMID 17280498.
- S2CID 8565063.
- ^ WO 93012096, Bridger G, Padmanabhan S, Skerlj RT, Thornton DM, "Linked cyclic polyamines with activity against HIV", published 24 June 1993, assigned to Johnson Matthey Public Limited Company
- .
- ^ .
- ^ "Mozobil approved for non-Hodgkin's lymphoma and multiple myeloma" (Press release). Monthly Prescribing Reference. 18 December 2008. Archived from the original on 6 January 2009. Retrieved 3 January 2009.
- ^ Notice of Compliance information
- PMID 15574767.
- PMID 20179352.
- PMID 19128793.
- S2CID 219285881.
- PMID 21193025.
