Polymer degradation
| Polymer science |
|---|
 |
Polymer degradation is the reduction in the physical properties of a polymer, such as strength, caused by changes in its chemical composition. Polymers and particularly plastics are subject to degradation at all stages of their product life cycle, including during their initial processing, use, disposal into the environment and recycling.[1] The rate of this degradation varies significantly; biodegradation can take decades, whereas some industrial processes can completely decompose a polymer in hours.
Technologies have been developed to both inhibit or promote degradation. For instance,
In general, the effects of heat, light, air and water are the most significant factors in the degradation of plastic polymers. The major chemical changes are

PP: polypropylene, PE: polyethylene, PVC: Polyvinyl chloride, PS: Polystyrene, PET: Polyethylene terephthalate
Susceptibility
Plastics exist in huge variety, however several types of
These plastics are all
Degradation during processing

Thermoplastic polymers (be they virgin or recycled) must be heated until molten to be formed into their final shapes, with processing temperatures anywhere between 150-320 °C (300–600 °F) depending on the polymer.
Polymers are often subject to more than one round of melt-processing, which can cumulatively advance degradation. Virgin plastic typically undergoes compounding to introduce additives such as dyes, pigments and stabilisers. Pelletised material prepared in this may also be pre-dried in an oven to remove trace moisture prior to its final melting and moulding into plastic items. Plastic which is recycled by simple re‑melting (mechanical recycling) will usually display more degradation than fresh material and may have poorer properties as a result.[3]
Thermal oxidation
Although oxygen levels inside processing equipment are usually low, it cannot be fully excluded and thermal-oxidation will usually take place more readily than degradation that is exclusively thermal (i.e. without air).
Thermal degradation
Heating polymers to a sufficiently high temperature can cause damaging chemical changes, even in the absence of oxygen. This usually starts with
Thermo-mechanical degradation
Molten polymers are non-Newtonian fluids with high viscosities, and the interaction between their thermal and mechanical degradation can be complex. At low temperatures, the polymer-melt is more viscous and more prone to mechanical degradation via shear stress. At higher temperatures, the viscosity is reduced, but thermal degradation is increased. Friction at points of high sheer can also cause localised heating, leading to additional thermal degradation.
Mechanical degradation can be reduced by the addition of lubricants, also referred to as processing aids or flow aids. These can reduce friction against the processing machinery but also between polymer chains, resulting in a decrease in melt-viscosity. Common agents are high-molecular-weight waxes (paraffin wax, wax esters, etc.) or metal stearates (i.e.zinc stearate).
In-service degradation

Most plastic items, like packaging materials, are used briefly and only once. These rarely experience polymer degradation during their service-lives. Other items experience only gradual degradation from the natural environment. Some plastic items, however, can experience long service-lives in aggressive environments, particularly those where they are subject to prolonged heat or chemical attack. Polymer degradation can be significant in these cases and, in practice, is often only held back by the use of advanced
The in-service degradation of mechanical properties is an important aspect which limits the applications of these materials. Polymer degradation caused by in-service degradation can cause life threatening accidents. In 1996, a baby was fed via a Hickman line and suffered an infection, when new connectors were used by a hospital. The reason behind this infection was the cracking and erosion of the pipes from the inner side due to contact with liquid media.[7]
Chlorine-induced cracking

Electronics
Plastics are used extensively in the manufacture of electrical items, such as
Galvanic action

Polymer degradation by
Degradation in the environment
Most plastics do not
Photo-oxidation
Photo-oxidation is the combined action of UV-light and oxygen and is the most significant factor in the weathering of plastics.
Hydrolysis
Polymers with an all-carbon backbone, such as polyolefins, are usually resistant to hydrolysis. Condensation polymers like polyesters,[31] polyamides, polyurethanes and polycarbonates can be degraded by hydrolysis of their carbonyl groups, to give lower molecular weight molecules. Such reactions are exceedingly slow at ambient temperatures, however, they remain a significant source of degradation for these materials, particularly in the marine environment.[32] Swelling caused by the absorption of minute amounts of water can also cause environmental stress cracking, which accelerates degradation.
Ozonolysis of rubbers
Polymers, which are not fully
Biological degradation
The major appeal of biodegradation is that, in theory, the polymer will be completely consumed in the environment without needing complex waste management and that the products of this will be non-toxic. Most
Oxidation can be caused by melt-processing or weathering in the environment. Oxidation may be intentionally accelerated by the addition of biodegradable additives. These are added to the polymer during compounding to improve the biodegradation of otherwise very resistant plastics. Similarly, biodegradable plastics have been designed which are intrinsically biodegradable, provided they are treated like compost and not just left in a landfill site where degradation is very difficult because of the lack of oxygen and moisture.[37]
Degradation during recycling

The act of recycling plastic degrades its polymer chains, usually as a result of thermal damage similar to that seen during initial processing. In some cases, this is turned into an advantage by intentionally and completely depolymerising the plastic back into its starting
Remelting
Thermoplastic polymers like polyolefins can be remelted and reformed into new items. This approach is referred to as mechanical recycling and is usually the simplest and most economical form of recovery.
Thermal depolymerisation & pyrolysis
As polymers approach their
Chemical depolymerisation
Stabilisers
Hindered amine light stabilizers (HALS) stabilise against weathering by scavenging
Detection
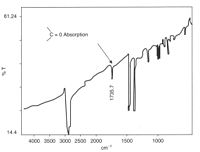
Degradation can be detected before serious cracks are seen in a product using infrared spectroscopy.[53] In particular, peroxy-species and carbonyl groups formed by photo-oxidation have distinct absorption bands.
See also
- Applied spectroscopy
- Forensic polymer engineering
- Environmental stress fracture
- Polymer engineering
- Weather testing of polymers
Bibliography
- Lewis, Peter Rhys, Reynolds, K and Gagg, C, Forensic Materials Engineering: Case studies, CRC Press (2004)
- Ezrin, Meyer, Plastics Failure Guide: Cause and Prevention, Hanser-SPE (1996).
- Wright, David C., Environmental Stress Cracking of Plastics RAPRA (2001).
- Lewis, Peter Rhys, and Gagg, C, Forensic Polymer Engineering: Why polymer products fail in service, Woodhead/CRC Press (2010).
References
- .
- PMID 28823699.
- ^ PMID 33000883.
- .
- PMID 26687228.
- .
- S2CID 98370832.
- ISBN 978-92-4-154995-0.
- .
- S2CID 137141131.
- S2CID 197611740.
- .
- .
- .
- .
- ISBN 0-938994-56-5.
- ^ C, Faudree M. (1991). "電気化学的過程とグラファイト/ポリイミド複合材料の関係". International Sampe Symposium and Exhibition (Society for the Advancement of Material and Process Engineering). 36 (2): 1288–1301.
- ISSN 1991-8178.
- ^ Dornheim, Michael (November 26, 1990). "ATF Researchers Address Potential for Bismaleimide Composile Degradation". Aviation Week and Science Technology: 122–123.
- ISBN 0-938994-56-5.
- .
- .
- .
- .
- .
- ^ .
- ^ S2CID 92300829.
- PMID 29035713.
- PMID 19528054.
- S2CID 96033161.
- .
- PMID 26216708.
- S2CID 3962436.
- PMID 31324632.
- PMID 19865515.
- S2CID 8797245.
- PMID 18337047.
- .
- PMID 19528059.
- .
- S2CID 209432804.
- .
- S2CID 199067235.
- .
- .
- .
- S2CID 215760966.
- .
- PMID 28371373.
- .
- S2CID 216028039.
- PMID 26482561.
- S2CID 233639741.


