Polypropylene

| |

| |

| |
| Names | |
|---|---|
| IUPAC name
Poly(1-methylethylene)
| |
| Other names
Polypropylene; Polypropene;
Polipropene 25 [USAN]; Propene polymers; Propylene polymers; 1-Propene; [-Ch2-Ch(Ch3)-]n | |
| Identifiers | |
| ChemSpider |
|
ECHA InfoCard
|
100.117.813 |
| UNII | |
CompTox Dashboard (EPA)
|
|
| Properties | |
| (C3H6)n | |
| Density | 0.855 g/cm3, amorphous 0.946 g/cm3, crystalline |
| Melting point | 130 to 171 °C (266 to 340 °F; 403 to 444 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Polypropylene (PP), also known as polypropene, is a
Polypropylene belongs to the group of polyolefins and is partially crystalline and non-polar. Its properties are similar to polyethylene, but it is slightly harder and more heat-resistant. It is a white, mechanically rugged material and has a high chemical resistance.[1]
Bio-PP is the
Polypropylene is the second-most widely produced commodity plastic (after polyethylene).
History
Chemical and physical properties
Polypropylene is in little aspects similar to
Mechanical properties
The density of PP is between 0.895 and 0.93 g/cm3. Therefore, PP is the commodity plastic with the lowest density. With lower density, moldings parts with lower weight and more parts of a certain mass of plastic can be produced. Unlike polyethylene, crystalline and amorphous regions differ only slightly in their density. However, the density of polyethylene can significantly change with fillers.[8]: 24
The Young's modulus of PP is between 1300 and 1800 N/mm².
Polypropylene is normally tough and flexible, especially when copolymerized with ethylene. This allows polypropylene to be used as an engineering plastic, competing with materials such as acrylonitrile butadiene styrene (ABS). Polypropylene is reasonably economical.[citation needed]
Polypropylene has good resistance to fatigue.[10]: 3070
Thermal properties
The melting point of polypropylene occurs in a range, so the melting point is determined by finding the highest temperature of a
The thermal expansion of PP is significant, but somewhat less than that of polyethylene. [11]
Chemical properties
Polypropylene at room temperature is resistant to fats and almost all organic
Most commercial polypropylene is
The melt flow rate (MFR) or melt flow index (MFI) is a measure of molecular weight of polypropylene. The measure helps to determine how easily the molten raw material will flow during processing. Polypropylene with higher MFR will fill the plastic mold more easily during the injection or blow-molding production process. As the melt flow increases, however, some physical properties, like impact strength, will decrease.
There are three general types of polypropylene:
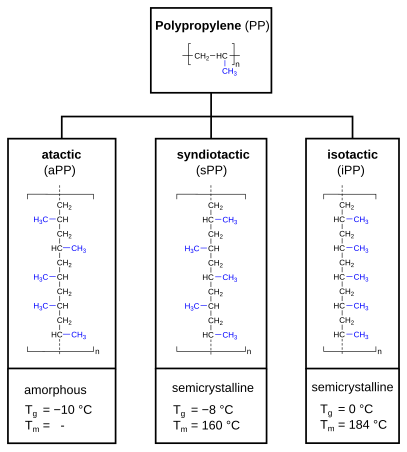
Molecular structure – tacticity
Polypropylene can be categorized as atactic polypropylene (aPP), syndiotactic polypropylene (sPP) and isotactic polypropylene (iPP). In case of atactic polypropylene, the methyl group (-CH3) is randomly aligned, alternating (alternating) for syndiotactic polypropylene and evenly for isotactic polypropylene. This has an impact on the crystallinity (amorphous or semi-crystalline) and the thermal properties (expressed as
The term
Atactic polypropylene, on the other hand, lacks any regularity which makes it unable to crystallize and amorphous.
Crystal structure of polypropylene
Isotactic polypropylene has a high degree of crystallinity, in industrial products 30–60%. Syndiotactic polypropylene is slightly less crystalline, atactic PP is amorphous (not crystalline).[13]: 251
Isotactic polypropylene (iPP)
Is polypropylene can exist in various crystalline modifications which differ by the molecular arrangement of the polymer chains. The crystalline modifications are categorized into the α-, β- and γ-modification as well as mesomorphic (smectic) forms.[14] The α-modifications is predominant in iPP. Such crystals are built from lamellae in the form of folded chains. A characteristic anomaly is that the lame are arranged in the so-called "cross-hatched" structure.[15] The melting point of α-crystalline regions is given as 185[16][17] to 220 °C,[16][18] the density as 0.936 to 0.946 g·cm−3.[19][20] The β-modification is in comparison somewhat less ordered, as a result of which it forms faster[21][22] and has a lower melting point of 170 to 200 °C.[16][23][24][18] The formation of the β-modification can be promoted by nucleating agents, suitable temperatures and shear stress.[21][25] The γ-modification is hardly formed under the conditions used in industry and is poorly understood. The mesomorphic modification, however, occurs often in industrial processing, since the plastic is usually cooled quickly. The degree of order of the mesomorphic phase ranges between the crystalline and the amorphous phase, its density is with 0.916 g·cm−3 comparatively. The mesomorphic phase is considered as cause for the transparency in rapidly cooled films (due to low order and small crystallites).[13]
Syndiotactic polypropylene (sPP)
Syndiotactic polypropylene was discovered much later than isotactic PP and could only be prepared by using
Atactic polypropylene (aPP)
Atactic polypropylene is amorphous and has therefore no crystal structure. Due to its lack of crystallinity, it is readily soluble even at moderate temperatures, which allows to separate it as by-product from isotactic polypropylene by extraction. However, the aPP obtained this way is not completely amorphous but can still contain 15% crystalline parts. Atactic polypropylene can also be produced selectively using metallocene catalysts, atactic polypropylene produced this way has a considerably higher molecular weight.[13]
Atactic polypropylene has lower density, melting point and softening temperature than the crystalline types and is tacky and rubber-like at room temperature. It is a colorless, cloudy material and can be used between −15 and +120 °C. Atactic polypropylene is used as a sealant, as an
Copolymers
Polypropylene
PP-RCT
Polypropylene random crystallinity temperature (PP-RCT), also used for plastic pipework, is a new form of this plastic. It achieves higher strength at high temperature by β-crystallization.[30]
Degradation

Polypropylene is liable to chain degradation from exposure to temperatures above 100 °C. Oxidation usually occurs at the
Microbial communities isolated from soil samples mixed with starch have been shown to be capable of degrading polypropylene.[31] Polypropylene has been reported to degrade while in the human body as implantable mesh devices. The degraded material forms a tree bark-like layer at the surface of mesh fibers.[32]
Optical properties
PP can be made
Production
Polypropylene is produced by the
The industrial production processes can be grouped into gas phase polymerization, bulk polymerization and slurry polymerization. All state-of-the-art processes use either gas-phase or bulk reactor systems.[1]
- In gas-phase and slurry-reactors, the polymer is formed around heterogeneous catalyst particles. The gas-phase polymerization is carried out in a propene is passed over a bed containing the heterogeneous (solid) catalyst and the formed polymer is separated as a fine powder and then converted into pellets. Unreacted gas is recycled and fed back into the reactor.
- In bulk polymerization, liquid propene acts as a solvent to prevent the precipitation of the polymer. The polymerization proceeds at 60 to 80 °C and 30–40 atm are applied to keep the propene in the liquid state. For the bulk polymerization, typically loop reactors are applied. The bulk polymerization is limited to a maximum of 5% ethene as comonomer due to a limited solubility of the polymer in the liquid propene.
- In the slurry polymerization, typically C4–C6 alkanes (butane, pentane or hexane) are utilized as inert diluent to suspend the growing polymer particles. Propene is introduced into the mixture as a gas.
The properties of PP are strongly affected by its tacticity, the orientation of the methyl groups (CH
3) relative to the methyl groups in neighboring monomer units (see above). The tacticity of polypropylene can be chosen by the choice of an appropriate catalyst.
Catalysts
The properties of PP are strongly affected by its tacticity, the orientation of the methyl groups (CH
3 in the figure) relative to the methyl groups in neighboring monomer units. A Ziegler–Natta catalyst is able to restrict linking of monomer molecules to a specific orientation, either isotactic, when all methyl groups are positioned at the same side with respect to the backbone of the polymer chain, or syndiotactic, when the positions of the methyl groups alternate. Commercially available isotactic polypropylene is made with two types of Ziegler-Natta catalysts. The first group of the catalysts encompasses solid (mostly supported) catalysts and certain types of soluble metallocene catalysts. Such isotactic macromolecules coil into a helical shape; these helices then line up next to one another to form the crystals that give commercial isotactic polypropylene many of its desirable properties.

Another type of metallocene catalysts produce syndiotactic polypropylene.[26] These macromolecules also coil into helices (of a different type) and crystallize. Atactic polypropylene is an amorphous rubbery material. It can be produced commercially either with a special type of supported Ziegler-Natta catalyst or with some metallocene catalysts.
Modern supported Ziegler-Natta catalysts developed for the polymerization of propylene and other
4 as an active ingredient and MgCl
2 as a support.[33][34][35]
Commercial synthesis of syndiotactic polypropylene is carried out with the use of a special class of metallocene catalysts. They employ bridged bis-metallocene complexes of the type bridge-(Cp1)(Cp2)ZrCl2 where the first Cp ligand is the cyclopentadienyl group, the second Cp ligand is the fluorenyl group, and the bridge between the two Cp ligands is -CH2-CH2-, >SiMe2, or >SiPh2.[36] These complexes are converted to polymerization catalysts by activating them with a special organoaluminum cocatalyst, methylaluminoxane (MAO).[37]
Manufacturing from polypropylene
Melting process of polypropylene can be achieved via extrusion and molding. Common extrusion methods include production of melt-blown and spun-bond fibers to form long rolls for future conversion into a wide range of useful products, such as face masks, filters, diapers and wipes.
The most common shaping technique is
The large number of end-use applications for polypropylene are often possible because of the ability to tailor grades with specific molecular properties and additives during its manufacture. For example,
Expanded Polypropylene (EPP) has been produced through both solid and melt state processing. EPP is manufactured using melt processing with either chemical or physical blowing agents. Expansion of PP in solid state, due to its highly crystalline structure, has not been successful. In this regard, two novel strategies were developed for expansion of PP. It was observed that PP can be expanded to make EPP through controlling its crystalline structure or through blending with other polymers.[38][39]
Biaxially oriented polypropylene (BOPP)
When polypropylene film is extruded and stretched in both the machine direction and across machine direction it is called biaxially oriented polypropylene. Two methods are widely used for producing BOPP films, namely, the tender process and tubular process.
Applications

As polypropylene is resistant to fatigue, most plastic living hinges, such as those on flip-top bottles, are made from this material. However, it is important to ensure that chain molecules are oriented across the hinge to maximise strength.
Polypropylene is used in the manufacturing of

Many plastic items for medical or laboratory use can be made from polypropylene because it can withstand the heat in an autoclave. Its heat resistance also enables it to be used as the manufacturing material of consumer-grade kettles[citation needed]. Food containers made from it will not melt in the dishwasher, and do not melt during industrial hot filling processes. For this reason, most plastic tubs for dairy products are polypropylene sealed with aluminum foil (both heat-resistant materials). After the product has cooled, the tubs are often given lids made of a less heat-resistant material, such as LDPE or polystyrene. Such containers provide a good hands-on example of the difference in modulus, since the rubbery (softer, more flexible) feeling of LDPE with respect to polypropylene of the same thickness is readily apparent. Rugged, translucent, reusable plastic containers made in a wide variety of shapes and sizes for consumers from various companies such as Rubbermaid and Sterilite are commonly made of polypropylene, although the lids are often made of somewhat more flexible LDPE so they can snap onto the container to close it. Polypropylene can also be made into disposable bottles to contain liquid, powdered, or similar consumer products, although HDPE and polyethylene terephthalate are commonly also used to make bottles. Plastic pails, car batteries, wastebaskets, pharmacy prescription bottles, cooler containers, dishes and pitchers are often made of polypropylene or HDPE, both of which commonly have rather similar appearance, feel, and properties at ambient temperature. An abundance of medical devices are made from PP.[47]
A common application for polypropylene is as biaxially oriented polypropylene (BOPP). These BOPP sheets are used to make a wide variety of materials including clear bags. When polypropylene is biaxially oriented, it becomes crystal clear and serves as an excellent packaging material for artistic and retail products.
Polypropylene, highly colorfast, is widely used in manufacturing carpets, rugs and mats to be used at home.[48]
Polypropylene is widely used in ropes, distinctive because they are light enough to float in water.[49] For equal mass and construction, polypropylene rope is similar in strength to polyester rope. Polypropylene costs less than most other synthetic fibers.
Polypropylene is also used as an alternative to
Polypropylene is also used in particular roofing membranes as the waterproofing top layer of single-ply systems as opposed to modified-bit systems.
Polypropylene is most commonly used for plastic moldings, wherein it is injected into a mold while molten, forming complex shapes at relatively low cost and high volume; examples include bottle tops, bottles, and fittings.
It can also be produced in sheet form, widely used for the production of stationery folders, packaging, and storage boxes. The wide color range, durability, low cost, and resistance to dirt make it ideal as a protective cover for papers and other materials. It is used in Rubik's Cube stickers because of these characteristics.
The availability of sheet polypropylene has provided an opportunity for the use of the material by designers. The light-weight, durable, and colorful plastic makes an ideal medium for the creation of light shades, and a number of designs have been developed using interlocking sections to create elaborate designs.
Polypropylene fibres are used as a
Clothing

Polypropylene is a major polymer used in
Polypropylene, or 'polypro', has been used for the fabrication of cold-weather base layers, such as long-sleeve shirts or long underwear. Polypropylene is also used in warm-weather clothing, in which it transports sweat away from the skin. Polyester has replaced polypropylene in these applications in the U.S. military, such as in the ECWCS.[52] Although polypropylene clothes are not easily flammable, they can melt, which may result in severe burns if the wearer is involved in an explosion or fire of any kind.[53] Polypropylene undergarments are known for retaining body odors which are then difficult to remove. The current generation of polyester does not have this disadvantage.[54]
Medical
Its most common medical use is in the synthetic, nonabsorbable
Polypropylene has been used in hernia and pelvic organ prolapse repair operations to protect the body from new hernias in the same location. A small patch of the material is placed over the spot of the hernia, below the skin, and is painless and rarely, if ever, rejected by the body. However, a polypropylene mesh will erode the tissue surrounding it over the uncertain period from days to years.
A notable application was as a transvaginal mesh, used to treat vaginal prolapse and concurrent urinary incontinence.[55] Due to the above-mentioned propensity for polypropylene mesh to erode the tissue surrounding it, the FDA has issued several warnings on the use of polypropylene mesh medical kits for certain applications in pelvic organ prolapse, specifically when introduced in close proximity to the vaginal wall due to a continued increase in number of mesh-driven tissue erosions reported by patients over the past few years.[56] On 3 January 2012, the FDA ordered 35 manufacturers of these mesh products to study the side effects of these devices. Due to the outbreak of the COVID-19 pandemic in 2020, the demand for PP has increased significantly because it's a vital raw material for producing meltblown fabric, which is in turn the raw material for producing facial masks.
Recycling
Polypropylene has "5" as its resin identification code:[57] 
Repairing
PP objects can be joined with a
PP can be melted using a
Health concerns
The advocacy organization Environmental Working Group classifies PP as of low to moderate hazard.[59][why?] PP is
Combustibility
Like all organic compounds, polypropylene is combustible.[61] The flash point of a typical composition is 260 °C; autoignition temperature is 388 °C.[62]
References
- ^ ISBN 978-3527306732.
- ^ Bio-based drop-in, smart drop-in and dedicated chemicals
- ^ wur.nl; Dutch, Duurzame bioplastics op basis van hernieuwbare grondstoffen
- .
- ISBN 978-0-941901-03-1.
- ^ "This week 50 years ago". New Scientist. 28 April 2007. p. 15.
- S2CID 253825228.
- ^ ISBN 978-1859572825.
- ^ "Polypropylene Plastic Materials & Fibers by Porex". www.porex.com. Archived from the original on 2016-11-14. Retrieved 2016-11-09.
- ^ ISBN 978-1-884207-58-7.
- ^ ISBN 978-3-446-43047-1.
- ISBN 978-3-642-34772-6.[page needed]
- ^ OCLC 706947259.
- .
- OCLC 441127075.[page needed]
- ^ .
- .
- ^ .
- .
- ISBN 978-0-471-53205-7.
- ^ .
- .
- S2CID 137665080.
- .
- .
- ^ .
- ISBN 978-0-8412-2456-8.
- .
- OCLC 213395068.
- ^ "Novinky v produkci FV Plast - od PP-R k PP-RCT". FV - Plast, a.s., Czech Republic. Archived from the original on 2019-11-30. Retrieved 2019-11-30.
- PMID 8285678.
- PMID 26315946.
- ISBN 978-0-444-53215-2.
- ISBN 978-0-470-13798-7.
- ISBN 1569902089.[page needed]
- ISBN 978-1-884207-59-4.[page needed]
- ^ Sinn, H.; Kaminsky, W.; Höker, H., eds. (1995). Alumoxanes, Macromol. Symp. 97. Heidelberg: Huttig & Wepf.[page needed]
- .
- .
- ^ "Polypropylene Applications". Euroshore. Retrieved 2021-05-25.
- ^ "Biaxially Oriented Polypropylene Films". Granwell. Retrieved 2012-05-31.
- ^ Neagu, Cristina (2020-02-05). "What is BOPP?". Sticker Mule. Retrieved 2021-06-25.
- ^ Green pipe helps miners remove the black Contractor Magazine, 10 January 2010
- ^ Contractor Retrofits His Business. the News/ 2 November 2009.
- ^ What to do when the piping replacement needs a replacement? Engineered Systems. 1 November 2009.
- S2CID 2648056.
- ^ Rug fibers Archived 2010-04-05 at the Wayback Machine. Fibersource.com. Retrieved on 2012-05-31.
- ^ Braided Polypropylene Rope is Inexpensive and it Floats. contractorrope.com. Retrieved on 2013-02-28.
- doi:10.14359/4439.
- S2CID 128478509.
- ^ Generation III Extended Cold Weather Clothing System (ECWCS). PM Soldier Equipment. October 2008
- ^ USAF Flying Magazine. Safety. Nov. 2002. access.gpo.gov
- ^ Ellis, David. Get Real: The true story of performance next to skin fabrics. outdoorsnz.org.nz
- S2CID 2648056.
- ^ UPDATE on Serious Complications Associated with Transvaginal Placement of Surgical Mesh for Pelvic Organ Prolapse: FDA Safety Communication, FDA, July 13, 2011
- ^ Plastics recycling information sheet Archived 2010-07-22 at the Wayback Machine, Waste Online
- ISBN 978-1-64429-251-8.
- ^ Polypropylene || Skin Deep Cosmetics Database | Environmental Working Group. Cosmeticdatabase.com. Retrieved on 2012-05-31.
- ^ Chapagain, A. K. et al. (September 2005) The water footprint of cotton consumption. UNESCO-IHE Delft. Value of Water Research Report Series No. 18. waterfootprint.org
- ISBN 978-94-010-5899-5.
- ^ "A&C Plastics Polypropylene MSDS" (PDF).