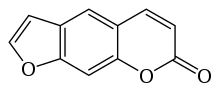
Psoralen
| |
| Names | |
|---|---|
| Preferred IUPAC name
7H-Furo[3,2-g][1]benzopyran-7-one | |
| Identifiers | |
3D model (
JSmol ) |
|
| 152784 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
ECHA InfoCard
|
100.000.581 |
| EC Number |
|
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C11H6O3 | |
| Molar mass | 186.16 g/mol |
| Melting point | 158 to 161 °C (316 to 322 °F; 431 to 434 K) |
| Hazards | |
| GHS labelling: | |

| |
| Warning | |
| H302, H315, H319, H335 | |
| P261, P264, P270, P271, P280, P301+P312, P302+P352, P304+P340, P305+P351+P338, P312, P321, P330, P332+P313, P337+P313, P362, P403+P233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Psoralen (also called psoralene) is the parent compound in a family of naturally occurring organic compounds known as the linear
Uses
Psoralen is a mutagen, and is used for this purpose in molecular biology research. Psoralen intercalates into DNA and on exposure to ultraviolet (UVA) radiation can form monoadducts and covalent interstrand cross-links (ICL) with thymines, preferentially at 5'-TpA sites in the genome, inducing apoptosis. Psoralen plus UVA (PUVA) therapy can be used to treat hyperproliferative skin disorders like psoriasis and certain kinds of skin cancer.[2] Unfortunately, PUVA treatment itself leads to a higher risk of skin cancer.[3]
An important use of psoralen is in PUVA treatment for skin problems such as psoriasis and, to a lesser extent, eczema and vitiligo. This takes advantage of the high UV absorbance of psoralen. The psoralen is applied first to sensitise the skin, then UVA light is applied to address the condition. Psoralens are also used in photopheresis, where they are mixed with the extracted leukocytes before UV radiation is applied.
Despite the photocarcinogenic properties of psoralen,[4][5] it was used as a tanning activator in sunscreens until 1996.[6] Psoralens are used in tanning accelerators, because psoralen increases the skin's sensitivity to light. Some patients have had severe skin loss after sunbathing with psoralen-containing tanning activators.[7] Patients with lighter skin colour suffer four times as much from the melanoma-generating properties of psoralens than those with darker skin.[6] Psoralens short term side effects include nausea, vomiting, erythrema, pruritus, xerosis, skin pain due to phototoxic damage of dermal nerve and may cause cutaneous and genital skin malignancies.[8]
An additional use for optimized psoralens is for the inactivation of pathogens in blood products. The synthetic amino-psoralen, amotosalen HCl, has been developed for the inactivation of infectious pathogens (bacteria, viruses, protozoa) in platelet and plasma blood components prepared for transfusion support of patients. Prior to clinical use, amotosalen-treated platelets have been tested and found to be non-carcinogenic when using the established p53 knockout mouse model.[9] The technology is currently in routine use in certain European blood centers and has been recently approved in the US.[10][11][12][13]
Chemistry
Psoralen intercalates into the DNA double helix where it is ideally positioned to form one or more adducts with adjacent pyrimidine bases, preferentially thymine, upon excitation by an ultraviolet photon.
Several physicochemical methods have been employed to derive binding constants for psoralen-DNA interactions. Classically, two chambers of psoralen and buffered DNA solution are partitioned by a
The photochemically reactive sites in psoralens are the alkene-like carbon-carbon double bonds in the furan ring (the five-member ring) and the pyrone ring (the six-member ring). When appropriately intercalated adjacent to a pyrimidine base, a four-center photocycloaddition reaction can lead to the formation of either of two cyclobutyl-type monoadducts. Ordinarily, furan-side monoadducts form in a higher proportion. The furan monoadduct can absorb a second UVA photon leading to a second four-center photocycloaddition at the pyrone end of the molecule and hence the formation of a diadduct or cross-link. Pyrone monoadducts do not absorb in the UVA range and hence cannot form cross-links with further UVA irradiation.[15]
Another important feature of this class of compounds is their ability to generate singlet oxygen, although this process is in direct competition with adduct formation and may be an alternate pathway for the dissipation of excited state energy.
Research on psoralen has historically focused on interactions with DNA and RNA (in particular, ICL formation). Psoralen, however, has also been shown to block signaling of the
Structure
Most furanocoumarins can be regarded as derivatives of either psoralen or angelicin. Psoralen and its derivatives are often referred to as the linear furanocoumarins, so called since they exhibit a linear chemical structure. Important linear furanocoumarins include xanthotoxin (also called methoxsalen), bergapten, imperatorin, and nodakenetin.

The structure of psoralen was originally deduced by identifying the products of its degradation reactions. It exhibits the normal reactions of the lactone of coumarin, such as ring opening by alkali to give a coumarinic acid or coumaric acid derivative. Potassium permanganate causes oxidation of the furan ring, while other methods of oxidation produce furan-2,3-carboxylic acid.
Synthesis
Psoralen is difficult to synthesize because

Biosynthesis
Psoralen originates from
A biosynthetic pathway in which psoralen is formed is shown in the figure below. A second

Plant sources
Repair of psoralen DNA adducts
The accurate process for repairing crosslinks is homologous recombinational repair (HRR). This involves replacing the damaged information using the intact information from another homologous chromosome in the same cell. Escherichia coli cells deficient in HRR are highly sensitive to PUVA compared to wild-type cells.[21] HRR appears to be efficient. In E. coli, even though one or two unrepaired crosslinks are sufficient to inactivate a cell, a wild-type cell can repair and therefore recover from 53 to 71 psoralen crosslinks.[21] In the yeast Saccharomyces cerevisiae HRR is a major pathway for accurately removing psoralen-crosslinks.[22] In wild-type yeast, the recombination events associated with crosslink removal by HRR are predominantly non-crossover gene conversion events. Psoralen crosslinks in virus DNA also appear to be removed by a recombinational repair process as occurs in SV40 virus infected cells,[23] and in herpes simplex virus infected cells.[24]
One inaccurate process for repairing psoralen crosslinks appears to employ a
Analysis of nucleic acids structures
Psoralens can reversibly crosslink nucleic acids double helices, and therefore have been used extensively for the analysis of interactions and structures for both DNA and RNA.[25][26]
References
- ^ Dean, F. M. (1963). Naturally occurring oxygen ring compounds. London: Butterworths.
- PMID 15891767.
- PMID 9589196.
- S2CID 4345680.
- PMID 7273295.
- ^ PMID 9233521.
- PMID 8982544.
- PMID 25382505.
- ^ Ciaravino V, McCullough T, Dayan AD: Pharmacokinetic and toxicology assessment of INTERCEPT (S-59 and UVA treated)platelets. Human Exp Toxicol 2001;20:533–550
- S2CID 25477437.
- S2CID 32119458.
- ^ "FDA approves first pathogen reduction system to treat plasma". www.fda.gov. Archived from the original on 2014-12-25.
- ^ "FDA approves pathogen reduction system to treat platelets". www.fda.gov. Archived from the original on 2014-12-25.
- S2CID 206270126.
- ISBN 978-0-8493-4379-7.)
{{cite book}}:|first1=has generic name (help)CS1 maint: multiple names: authors list (link - PMID 24551203.
- S2CID 6931552.
- ^ ISBN 978-0-471-49641-0.
- ^ "Dr. Duke's Phytochemical and Ethnobotanical Databases". U.S. Department of Agriculture, Agricultural Research Service. Retrieved October 6, 2018.
- PMID 785009.
- ^ PMID 361714.
- PMID 24969513.
- S2CID 5843939.
- PMID 6272987.
- PMID 2411210.
- PMID 27180905.
Further reading
- Dean, F.M. (1963). Naturally Occurring Oxygen Ring Compounds. London: Butterworths.
- The Merck Index (7th ed.). Rahway NJ: Merck. 1960.
