Q fever
| Q fever | |
|---|---|
| Other names | Query fever, coxiellosis Monoclonal antibodies against C. burnetii and hematoxylin were used for staining; original magnification is ×50. |
| Specialty | Infectious diseases |
| Types | acute, chronic[1] |
| Risk factors | Contact with livestock[2] |
| Differential diagnosis | pneumonia, influenza, brucellosis, leptospirosis, meningitis, viral hepatitis, dengue fever, malaria, other rickettsial infections[2] |
Q fever or query fever is a disease caused by infection with
Signs and symptoms
The incubation period is usually two to three weeks.
During its course, the disease can progress to an atypical pneumonia, which can result in a life-threatening acute respiratory distress syndrome, usually occurring during the first four to five days of infection.[7]
Less often, Q fever causes (granulomatous) hepatitis, which may be asymptomatic or become symptomatic with malaise, fever, liver enlargement, and pain in the right upper quadrant of the abdomen. This hepatitis often results in the elevation of transaminase values, although jaundice is uncommon. Q fever can also rarely result in retinal vasculitis.[8]
The chronic form of Q fever is virtually identical to endocarditis (i.e. inflammation of the inner lining of the heart),[9] which can occur months or decades following the infection. It is usually fatal if untreated. However, with appropriate treatment, the mortality falls to around 10%.[citation needed]
A minority of Q fever survivors develops Q fever fatigue syndrome after acute infection, one of the more well-studied post-acute infection syndromes. Q fever fatigue syndrome is characterised by post-exertional malaise and debilitating fatigue. People with Q fever fatigue syndrome frequently meet the diagnostic criteria for myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). Symptoms often persist years after the initial infection.[10]
Diagnosis
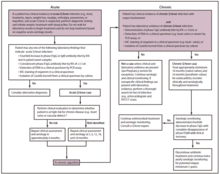
Diagnosis is usually based on
Q fever can cause endocarditis (infection of the heart valves) which may require transoesophageal echocardiography to diagnose. Q fever hepatitis manifests as an elevation of alanine transaminase and aspartate transaminase, but a definitive diagnosis is only possible on liver biopsy, which shows the characteristic fibrin ring granulomas.[14]
Prevention
Research done in the 1960s–1970s by French Canadian-American microbiologist and virologist Paul Fiset was instrumental in the development of the first successful Q fever vaccine.[15]
Protection is offered by Q-Vax, a whole-cell, inactivated vaccine developed by an Australian vaccine manufacturing company, CSL Limited.[16] The intradermal vaccination is composed of killed C. burnetii organisms. Skin and blood tests should be done before vaccination to identify pre-existing immunity, because vaccinating people who already have immunity can result in a severe local reaction. After a single dose of vaccine, protective immunity lasts for many years. Revaccination is not generally required. Annual screening is typically recommended.[17]
In 2001, Australia introduced a national Q fever vaccination program for people working in "at risk" occupations. Vaccinated or previously exposed people may have their status recorded on the Australian Q Fever Register,[18] which may be a condition of employment in the meat processing industry or in veterinary research.[19] An earlier killed vaccine had been developed in the Soviet Union, but its side effects prevented its licensing abroad.[citation needed]
Preliminary results suggest vaccination of animals may be a method of control. Published trials proved that use of a registered phase vaccine (Coxevac) on infected farms is a tool of major interest to manage or prevent early or late abortion, repeat breeding,
Treatment
Treatment of acute Q fever with
Epidemiology

Q fever is a globally distributed zoonotic disease caused by a highly sustainable and virulent bacterium. The pathogenic agent is found worldwide, with the exception of New Zealand[24] and Antarctica.[25] Understanding the transmission and risk factors of Q fever is crucial for public health due to its potential to cause widespread infection.
Transmission and occupational risks
Transmission primarily occurs through the inhalation of contaminated dust, contact with contaminated milk, meat, or wool, and particularly birthing products.
- Veterinarypersonnel
- Stockyard workers
- Farmers
- Sheep shearers
- Animal transporters
- Laboratory workers handling potentially infected veterinary samples or visiting abattoirs
- People who cull and process kangaroos
- Hide (tannery) workers
It is important to note that anyone who has contact with animals infected with Q fever bacteria, especially people who work on farms or with animals, is at an increased risk of contracting the disease.[27] Understanding these occupational risks is crucial for public health.
Prevalence and risk factors
Studies indicate a higher prevalence of Q fever in men than in women,[28][29] potentially linked to occupational exposure rates.[30] Other contributing risk factors include geography, age, and occupational exposure. Diagnosis relies on blood compatibility testing, with treatment varying for acute and chronic cases. Acute disease often responds to doxycycline, while chronic cases may require a combination of doxycycline and hydroxychloroquine.[31] It is worth noting that Q fever was officially reported in the United States as a notifiable disease in 1999 due to its potential biowarfare agent status.[32]
Q fever exhibits global epidemiological patterns, with higher incidence rates reported in certain countries. In Africa, wild animals in rainforests primarily transmit the disease, making it endemic.[25] Unique patterns are observed in Latin America, but reporting is sporadic and inconsistent between and among countries, making it difficult to track and address.[33]
Recent outbreaks in European countries, including the Netherlands and France, have been linked to urbanized goat farming, raising concerns about the safety of intensive livestock farming practices and the potential risks of zoonotic diseases. Similarly, in the United States, Q fever is more common in livestock farming regions, especially in the West and the Great Plains. California, Texas, and Iowa account for almost 40% of reported cases, with a higher incidence during the spring and early summer when livestock are breeding, regardless of whether the infection is acute or chronic.[27]
These outbreaks have affected a significant number of people, with immunocompromised individuals being more severely impacted.[32] The global nature of Q fever and its association with livestock farming highlight the importance of implementing measures to prevent and control the spread of the disease, particularly in high-risk regions.
Age and occupational exposure
Older men in the West and Great Plains regions, involved in close contact with livestock management, are at a higher risk of contracting chronic Q fever.[30] This risk may be further increased for those with a history of cardiac problems.[30] The disease can manifest years after the initial infection, presenting symptoms such as non-specific fatigue, fever, weight loss, and endocarditis.[25][30] Additionally, certain populations have been found to be more vulnerable to Q fever, including children living in farming communities, who may experience similar symptoms as adults.[34] There have also been reported cases of Q fever among United States military service members, particularly those deployed to Iraq or Afghanistan, which further highlights the importance of understanding and addressing the occupational risks associated with Q fever.[35]
Prevention and public health education
Proper public health education is crucial in reducing the number of Q fever cases. Raising awareness about transmission routes, occupational risks, and preventive measures,[32] such as eliminating unpasteurized milk products from the diet, can help prevent the spread of disease.[36]
Interdisciplinary collaboration between medical personnel and farmers is critical when developing strategies for control and prevention in a community.[37] Awareness campaigns should particularly target occupations that work with livestock, focusing on risk-reduction procedures such as herd monitoring, implementing sanitation practices and personal protective equipment, and vaccinating animals.[37] Locating livestock farms at least 500 meters away from residential areas can also help reduce animal-to-human transmission.[37]
History
Q fever was first described in 1935 by Edward Holbrook Derrick[38] in slaughterhouse workers in Brisbane, Queensland. The "Q" stands for "query" and was applied at a time when the causative agent was unknown; it was chosen over suggestions of abattoir fever and Queensland rickettsial fever, to avoid directing negative connotations at either the cattle industry or the state of Queensland.[39]
The
Society and culture
An early mention of Q fever was important in one of the early Dr. Kildare films (1939, Calling Dr. Kildare). Kildare's mentor Dr. Gillespie (Lionel Barrymore) tires of his protégé working fruitlessly on "exotic diagnoses" ("I think it's Q fever!") and sends him to work in a neighborhood clinic, instead.[42][43]
Q fever was also highlighted in an episode of the U.S. television medical drama House ("The Dig", season seven, episode 18).[citation needed]
Biological warfare
C. burnetii has been used to develop biological weapons.[44]
The United States investigated it as a potential biological warfare agent in the 1950s, with eventual standardization as agent OU. At Fort Detrick and Dugway Proving Ground, human trials were conducted on Whitecoat volunteers to determine the median infective dose (18 MICLD50/person i.h.) and course of infection. The Deseret Test Center dispensed biological Agent OU with ships and aircraft, during Project 112 and Project SHAD.[45] As a standardized biological, it was manufactured in large quantities at Pine Bluff Arsenal, with 5,098 gallons in the arsenal in bulk at the time of demilitarization in 1970.[citation needed]
C. burnetii is currently ranked as a "category B" bioterrorism agent by the CDC.[46] It can be contagious, and is very stable in aerosols in a wide range of temperatures. Q fever microorganisms may survive on surfaces up to 60 days. It is considered a good agent in part because its ID50 (number of bacilli needed to infect 50% of individuals) is considered to be one, making it the lowest known.[dubious ]
In animals
Q fever can affect many species of domestic and wild animals, including ruminants (cattle, sheep, goats, bison,
Clinical signs
In contrast to humans, though a respiratory and cardiac infection could be experimentally reproduced in cattle,[54] the clinical signs mainly affect the reproductive system. Q fever in ruminants is, therefore, mainly responsible for abortions, metritis, retained placenta, and infertility.
The clinical signs vary between species. In small
In cattle, although abortions also occur, they are less frequent and more sporadic. The clinical picture is rather dominated by nonspecific signs such as placental retentions, metritis, and consequent fertility disorders.[56][57][58]
Epidemiology
With the exception of New Zealand, which is currently free of Q fever, the disease is present throughout the world. Numerous epidemiological surveys have been carried out. They have shown that about one in three cattle farms and one in four sheep or goat farms are infected,[59] but wide variations are seen between studies and countries. In China, Iran, Great Britain, Germany, Hungary, the Netherlands, Spain, the US, Belgium, Denmark, Croatia, Slovakia, the Czech Republic, Serbia, Slovenia, and Jordan, for example, more than 50% of cattle herds were infected with Q fever.[60][61][62][63][64][65][66]
Infected animals shed the bacteria by three routes - genital discharge, faeces, and milk.[67] Excretion is greatest at the time of parturition or abortion, and placentas and aborted fetuses are the main sources of bacteria, particularly in goats.
As C. burnetii is small and resistant in the environment, it is easily airborne and can be transmitted from one farm to another, even if several kilometres away.[68]
Control
Biosecurity measures
Based on the epidemiological data, biosecurity measures can be derived:[69]
- The spread of manure from infected farm should be avoided in windy conditions
- The level of hygiene must be very high during parturition and fetal annexes, and fetuses must be collected and destroyed as soon as possible
Medical measures
A vaccine for cattle, goats and sheep exists. It reduces clinical expression such as abortions and decreases excretion of the bacteria by the animals leading to control of Q fever in herds.[70]
In addition, vaccination of herds against Q fever has been shown to reduce the risk of human infection.[71]
References
- ^ a b c "Epidemiology and Statistics | Q Fever | CDC". www.cdc.gov. 2019-09-16. Archived from the original on 2020-05-29. Retrieved 2020-05-27.
- ^ ISBN 978-0-7817-3063-1.
- PMID 16547017.
- ^ "Q fever | Genetic and Rare Diseases Information Center (GARD) – an NCATS Program". Genetic and Rare Diseases Information Center. Archived from the original on 2018-04-18. Retrieved 2018-04-17.
- ^ a b "Q Fever | CDC". Centers for Disease Control and Prevention. 2017-12-27. Archived from the original on 2018-04-18. Retrieved 2018-04-17.
- ^ ISBN 978-0-19-976901-8.
- ^ a b c d "Coxiella/Q Fever". The Lecturio Medical Concept Library. Archived from the original on 2021-05-15. Retrieved 7 July 2021.
- PMID 1430809.
- PMID 16757641.
- S2CID 248889597.
- PMID 10515901.
- PMID 12491231.
- PMID 19246385.
- doi:10.1086/501027.
- ^ Saxon W (March 8, 2001). "Dr. Paul Fiset, 78, Microbiologist And Developer of Q Fever Vaccine". The New York Times. p. C-17. Archived from the original on 2015-05-27. Retrieved 2022-01-28.
- ^ "Q fever Vaccine" (PDF). CSL Limited. 17 January 2014. Archived from the original (PDF) on 9 March 2017. Retrieved 11 July 2015.
- ^ "USCF communicable disease prevention program animal exposure surveillance program" (PDF). Archived from the original (PDF) on 2007-07-01. Retrieved 2007-05-08.
- ^ "Australian Q Fever Register". AusVet. Archived from the original on 2016-02-16. Retrieved 12 February 2016.
- The University of Melbourne. 2019-11-20. Archivedfrom the original on 2019-06-25. Retrieved 2020-07-11.
- ^ Camuset P, Remmy D (2008). Q Fever (Coxiella burnetii) Eradication in a Dairy Herd by Using Vaccination with a Phase 1 Vaccine. XXV World Buiatrics Congress. Budapest.
- PMID 21392427.
- PMID 17682987.
- ^ "Query Fever - MARI REF". Misdiagnosis Association and Research Institute. 20 July 2021. Archived from the original on 2021-08-27. Retrieved 2021-08-27.
- PMID 17147957.
- ^ S2CID 73461378.
- ^ "Q fever: MedlinePlus Medical Encyclopedia". MedlinePlus. Archived from the original on 2016-07-28. Retrieved 2018-04-17.
- ^ a b CDC (2021-08-06). "Q fever epidemiology and statistics | CDC". Centers for Disease Control and Prevention. Retrieved 2023-11-21.
- PMID 10589906.
- PMID 3301708.
- ^ a b c d "Epidemiology and Statistics | Q Fever | CDC". www.cdc.gov. 2019-09-16. Archived from the original on 2020-05-29. Retrieved 2020-05-27.
- PMID 18452690.
- ^ PMID 32310555, retrieved 2023-11-21
- PMID 36915670.
- S2CID 253662234.
- PMID 18452690.
- ^ CDC (2019-01-15). "Prevention of Q fever | CDC". Centers for Disease Control and Prevention. Retrieved 2023-12-12.
- ^ PMID 36013948.
- .
- ISBN 978-0-8493-5984-2.
- PMID 6194551.
- PMID 19315693.
- ^ "Calling Dr. Kildare". Movie Mirrors Index. Archived from the original on 2012-02-04. Retrieved 30 April 2013.
- (PDF) from the original on 2010-11-30.
- PMID 14592601.
- ^ "Deseret Test Center, Project SHAD, Shady Grove revised fact sheet".
- PMID 12704232.
- PMID 34736464.
- PMID 22172398.
- PMID 22989183.
- PMID 33583452.
- S2CID 247236730.
- PMID 26134933.
- PMID 15845229.
- OCLC 862686843.
- PMID 15845229.
- S2CID 4268347.
- ^ Valla G. "Prevalenza di Coxiella burnetii nel latte di massa in allevamenti di bovine da latte italiani e possibile correlazione con problemi riproduttivi" (PDF).
- OCLC 836117348.
- PMID 21115308.
- PMID 30681125.
- ISSN 0924-2244.
- S2CID 226269478.
- S2CID 243762186.
- PMID 28272921.
- PMID 34068431.
- PMID 25056416.
- PMID 17903418.
- PMID 9782633.
- PMID 30084178.
- ^ "Coxevac | European Medicines Agency". 17 September 2018.
- S2CID 29026616.
