Quantum dot
| Part of a series of articles on |
| Nanomaterials |
|---|
 |
| Carbon nanotubes |
| Fullerenes |
| Other nanoparticles |
| Nanostructured materials |
Quantum dots (QDs) or semiconductor nanocrystals are
Nanoscale semiconductor materials tightly confine either electrons or
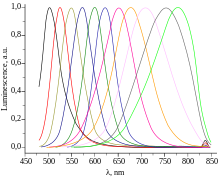
Quantum dots have properties intermediate between bulk semiconductors and discrete atoms or molecules. Their optoelectronic properties change as a function of both size and shape.[9][10] Larger QDs of 5–6 nm diameter emit longer wavelengths, with colors such as orange, or red. Smaller QDs (2–3 nm) emit shorter wavelengths, yielding colors like blue and green. However, the specific colors vary depending on the exact composition of the QD.[11]
Potential applications of quantum dots include
Core/shell and core/double-shell structures
Quantum dots are usually coated with organic capping ligands (typically with long hydrocarbon chains, such as oleic acid) to control growth, prevent aggregation, and to promote dispersion in solution.
Type I quantum dots are composed of a semiconductor core encapsulated in a second semiconductor material with a larger bandgap, which can passivate non-radiative recombination sites at the surface of the quantum dots and improve quantum yield. Inverse type I quantum dots have a semiconductor layer with a smaller bandgap which leads to delocalized charge carriers in the shell. For type II and inverse type II dots, either the conduction or valence band of the core is located within the bandgap of the shell, which can lead to spatial separation of charge carriers in the core and shell.[27] For all of these core/shell systems, the deposition of the outer layer can lead to potential lattice mismatch, which can limit the ability to grow a thick shell without reducing photoluminescent performance.
One such reason for the decrease in performance can be attributed to the physical strain being put on the lattice. In a case where ZnSe/ZnS (type I) and ZnSe/CdS (type II) quantum dots were being compared, the diameter of the uncoated ZnSe core (obtained using TEM) was compared to the capped core diameter (calculated via effective mass approximation model) [lattice strain source] to better understand the effect of core-shell strain.[28] Type I heterostructures were found to induce compressive strain and “squeeze” the core, while the type II heterostructures had the effect of stretching the core under tensile strain.[28] Because the fluorescent properties of quantum dots are dictated by nanocrystal size, induced changes in core dimensions can lead to shifting of emission wavelength, further proving why an intermediate semiconductor layer is necessary to rectify lattice mismatch and improve quantum yield.[29]
One such core/double-shell system is the CdSe/ZnSe/ZnS nanocrystal.[29] In a study comparing CdSe/ZnS and CdSe/ZnSe nanocrystals, the former was found to have PL yield 84% of the latter’s, due to a lattice mismatch. To study the double-shell system, after synthesis of the core CdSe nanocrystals, a layer of ZnSe was coated prior to the ZnS outer shell, leading to an improvement in fluorescent efficiency by 70%. Furthermore, the two additional layers were found to improve resistance of the nanocrystals against photo-oxidation, which can contribute to degradation of the emission spectra.
It is also standard for surface passivation techniques to be applied to these core/double-shell systems, as well. As mentioned above, oleic acid is one such organic capping ligand that is used to promote colloidal stability and control nanocrystal growth, and can even be used to initiate a second round of ligand exchange and surface functionalization.[24][30] However, because of the detrimental effect organic ligands have on PL efficiency, further studies have been conducted to obtain all-inorganic quantum dots. In one such study, intensely luminescent all-inorganic nanocrystals (ILANs) were synthesized via a ligand exchange process which substituted metal salts for the oleic acid ligands, and were found to have comparable photoluminescent quantum yields to that of existing red- and green-emitting quantum dots.[24]
Production

There are several ways to fabricate quantum dots. Possible methods include colloidal synthesis, self-assembly, and electrical gating.
Colloidal synthesis
There are colloidal methods to produce many different semiconductors. Typical dots are made of binary compounds such as lead sulfide, lead selenide, cadmium selenide, cadmium sulfide, cadmium telluride, indium arsenide, and indium phosphide. Dots may also be made from ternary compounds such as cadmium selenide sulfide. Further, recent advances have been made which allow for synthesis of colloidal perovskite quantum dots.[31] These quantum dots can contain as few as 100 to 100,000 atoms within the quantum dot volume, with a diameter of approximately 10 to 50 atom diameters. This corresponds to about 2 to 10
Large batches of quantum dots may be synthesized via
Plasma synthesis
Plasma synthesis has evolved to be one of the most popular gas-phase approaches for the production of quantum dots, especially those with covalent bonds.[32][33][34] For example, silicon and germanium quantum dots have been synthesized by using nonthermal plasma. The size, shape, surface and composition of quantum dots can all be controlled in nonthermal plasma.[35][36] Doping that seems quite challenging for quantum dots has also been realized in plasma synthesis.[37][38][39] Quantum dots synthesized by plasma are usually in the form of powder, for which surface modification may be carried out. This can lead to excellent dispersion of quantum dots in either organic solvents[40] or water[41] (i. e., colloidal quantum dots).
Fabrication
The
- Some quantum dots are small regions of one material buried in another with a larger
- Quantum dots sometimes occur spontaneously in quantum well structures due to monolayer fluctuations in the well's thickness.

- Self-assembled quantum dots nucleate spontaneously under certain conditions during quantum computation. The main limitations of this method are the cost of fabrication and the lack of control over positioning of individual dots.
- Individual quantum dots can be created from two-dimensional electron or hole gases present in remotely doped quantum wells or semiconductor heterostructures called electron beam lithography. This pattern can then be transferred to the electron or hole gas by etching, or by depositing metal electrodes (lift-off process) that allow the application of external voltages between the electron gas and the electrodes. Such quantum dots are mainly of interest for experiments and applications involving electron or hole transport and they are also used as spin qubits.[47]A strength of this type of quantum dots is that their energy spectrum can be engineered by controlling the geometrical size, shape, and the strength of the confinement potential with gate electrodes. These quantum dots can be easily connected by tunnel barriers to conducting leads, which allows the application of the techniques of tunneling spectroscopy for their investigation.
- silicon quantum dots. Ultra small (20 nm × 20 nm) CMOS transistors behave as single electron quantum dots when operated at cryogenic temperature over a range of −269 °C (4 K) to about −258 °C (15 K). The transistor displays Coulomb blockade due to progressive charging of electrons (holes) one by one. The number of electrons (holes) confined in the channel is driven by the gate voltage, starting from an occupation of zero electrons (holes), and it can be set to one or many.[48]
Viral assembly
Genetically engineered
Electrochemical assembly
Highly ordered arrays of quantum dots may also be self-assembled by
Bulk manufacture
Quantum dot manufacturing relies on a process called high temperature dual injection which has been scaled by multiple companies for commercial applications that require large quantities (hundreds of kilograms to tons) of quantum dots. This reproducible production method can be applied to a wide range of quantum dot sizes and compositions.
The bonding in certain cadmium-free quantum dots, such as
Another approach for the mass production of colloidal quantum dots can be seen in the transfer of the well-known hot-injection methodology for the synthesis to a technical continuous flow system. The batch-to-batch variations arising from the needs during the mentioned methodology can be overcome by utilizing technical components for mixing and growth as well as transport and temperature adjustments. For the production of CdSe based semiconductor nanoparticles this method has been investigated and tuned to production amounts of kilograms per month. Since the use of technical components allows for easy interchange in regards of maximum throughput and size, it can be further enhanced to tens or even hundreds of kilograms.[52]
In 2011 a consortium of U.S. and Dutch companies reported a milestone in high-volume quantum dot manufacturing by applying the traditional high temperature dual injection method to a flow system.[53]
On 23 January 2013 Dow entered into an exclusive licensing agreement with UK-based Nanoco for the use of their low-temperature molecular seeding method for bulk manufacture of cadmium-free quantum dots for electronic displays, and on 24 September 2014 Dow commenced work on the production facility in South Korea capable of producing sufficient quantum dots for "millions of cadmium-free televisions and other devices, such as tablets". Mass production is due to commence in mid-2015.[54] On 24 March 2015, Dow announced a partnership deal with LG Electronics to develop the use of cadmium free quantum dots in displays.[55]
Heavy-metal-free quantum dots
In many[
For commercial viability, a range of restricted, heavy-metal-free quantum dots has been developed showing bright emissions in the visible and near-infrared region of the spectrum and have similar optical properties to those of CdSe quantum dots. [citation needed] Among these materials are InP/ZnS, CuInS/ZnS,[clarification needed] Si, Ge, and C.
Peptides are being researched as potential quantum dot material.[56]
Health and safety
Some quantum dots pose risks to human health and the environment under certain conditions.
Many studies have focused on the mechanism of QD cytotoxicity using model cell cultures. It has been demonstrated that after exposure to ultraviolet radiation or oxidation by air, CdSe QDs release free cadmium ions causing cell death.[60] Group II–VI QDs also have been reported to induce the formation of reactive oxygen species after exposure to light, which in turn can damage cellular components such as proteins, lipids, and DNA.[61] Some studies have also demonstrated that addition of a ZnS shell inhibits the process of reactive oxygen species in CdSe QDs. Another aspect of QD toxicity is that there are, in vivo, size-dependent intracellular pathways that concentrate these particles in cellular organelles that are inaccessible by metal ions, which may result in unique patterns of cytotoxicity compared to their constituent metal ions.[62] The reports of QD localization in the cell nucleus[63] present additional modes of toxicity because they may induce DNA mutation, which in turn will propagate through future generation of cells, causing diseases.
Although concentration of QDs in certain organelles have been reported in in vivo studies using animal models, no alterations in animal behavior, weight, hematological markers, or organ damage has been found through either histological or biochemical analysis.[64] These findings have led scientists to believe that intracellular dose is the most important determining factor for QD toxicity. Therefore, factors determining the QD endocytosis that determine the effective intracellular concentration, such as QD size, shape, and surface chemistry determine their toxicity. Excretion of QDs through urine in animal models also have demonstrated via injecting radio-labeled ZnS-capped CdSe QDs where the ligand shell was labeled with 99mTc.[65] Though multiple other studies have concluded retention of QDs in cellular levels,[59][66] exocytosis of QDs is still poorly studied in the literature.
While significant research efforts have broadened the understanding of toxicity of QDs, there are large discrepancies in the literature, and questions still remain to be answered. Diversity of this class of material as compared to normal chemical substances makes the assessment of their toxicity very challenging. As their toxicity may also be dynamic depending on the environmental factors such as pH level, light exposure, and cell type, traditional methods of assessing toxicity of chemicals such as LD50 are not applicable for QDs. Therefore, researchers are focusing on introducing novel approaches and adapting existing methods to include this unique class of materials.[59] Furthermore, novel strategies to engineer safer QDs are still under exploration by the scientific community. A recent novelty in the field is the discovery of carbon quantum dots, a new generation of optically active nanoparticles potentially capable of replacing semiconductor QDs, but with the advantage of much lower toxicity.
Optical properties
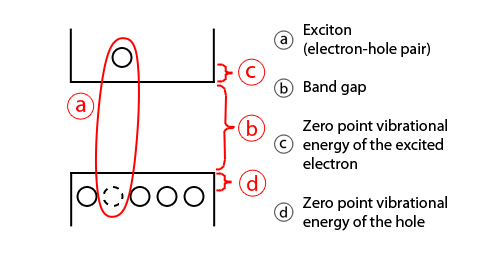
In semiconductors, light absorption generally leads to an electron being excited from the valence to the conduction band, leaving behind a hole. The electron and the hole can bind to each other to form an exciton. When this exciton recombines (when the electron resumes its ground state), the exciton's energy can be emitted as light. This is called fluorescence. In a simplified model, the energy of the emitted photon can be understood as the sum of the band gap energy between the highest occupied level and the lowest unoccupied energy level, the confinement energies of the hole and the excited electron, and the bound energy of the exciton (the electron–hole pair):
As the confinement energy depends on the quantum dot's size, both absorption onset and fluorescence emission can be tuned by changing the size of the quantum dot during its synthesis. The larger the dot, the redder (lower-energy) its absorption onset and fluorescence spectrum. Conversely, smaller dots absorb and emit bluer (higher-energy) light. Recent articles suggest that the shape of the quantum dot may be a factor in the coloration as well, but as yet not enough information is available [citation needed]. Furthermore, it was shown[67] that the lifetime of fluorescence is determined by the size of the quantum dot. Larger dots have more closely spaced energy levels in which the electron–hole pair can be trapped. Therefore, electron–hole pairs in larger dots live longer causing larger dots to show a longer lifetime.
To improve fluorescence
Applications
Quantum dots are particularly promising for optical applications due to their high
Tuning the size of quantum dots is attractive for many potential applications. For instance, larger quantum dots have a greater spectrum shift toward red compared to smaller dots and exhibit less pronounced quantum properties. Conversely, the smaller particles allow one to take advantage of more subtle quantum effects.
Being
CdSe nanocrystals are efficient triplet photosensitizers.[76] Laser excitation of small CdSe nanoparticles enables the extraction of the excited state energy from the quantum dots into bulk solution, thus opening the door to a wide range of potential applications such as photodynamic therapy, photovoltaic devices, molecular electronics, and catalysis.
Biology
In modern biological analysis, various kinds of organic dyes are used. However, as technology advances, greater flexibility in these dyes is sought.[77] To this end, quantum dots have quickly filled in the role, being found to be superior to traditional organic dyes on several counts, one of the most immediately obvious being brightness (owing to the high extinction coefficient combined with a comparable quantum yield to fluorescent dyes[17]) as well as their stability (allowing much less photobleaching).[78] It has been estimated that quantum dots are 20 times brighter and 100 times more stable than traditional fluorescent reporters.[77] For single-particle tracking, the irregular blinking of quantum dots is a minor drawback. However, there have been groups which have developed quantum dots which are essentially nonblinking and demonstrated their utility in single-molecule tracking experiments.[79][80]
The use of quantum dots for highly sensitive cellular imaging has seen major advances.
Quantum dots can have antibacterial properties similar to nanoparticles and can kill bacteria in a dose-dependent manner.[90] One mechanism by which quantum dots can kill bacteria is through impairing the functions of antioxidative system in the cells and down regulating the antioxidative genes. In addition, quantum dots can directly damage the cell wall. Quantum dots have been shown to be effective against both gram- positive and gram-negative bacteria.[91]
Semiconductor quantum dots have also been employed for
.One application of quantum dots in biology is as donor fluorophores in Förster resonance energy transfer, where the large extinction coefficient and spectral purity of these fluorophores make them superior to molecular fluorophores[92] It is also worth noting that the broad absorbance of QDs allows selective excitation of the QD donor and a minimum excitation of a dye acceptor in FRET-based studies.[93] The applicability of the FRET model, which assumes that the Quantum Dot can be approximated as a point dipole, has recently been demonstrated[94]
The use of quantum dots for tumor targeting under in vivo conditions employ two targeting schemes: active targeting and passive targeting. In the case of active targeting, quantum dots are functionalized with tumor-specific binding sites to selectively bind to tumor cells. Passive targeting uses the enhanced permeation and retention of tumor cells for the delivery of quantum dot probes. Fast-growing tumor cells typically have more permeable membranes than healthy cells, allowing the leakage of small nanoparticles into the cell body. Moreover, tumor cells lack an effective lymphatic drainage system, which leads to subsequent nanoparticle accumulation.
Quantum dot probes exhibit in vivo toxicity. For example, CdSe nanocrystals are highly toxic to cultured cells under UV illumination, because the particles dissolve, in a process known as
In another potential application, quantum dots are being investigated as the inorganic fluorophore for intra-operative detection of tumors using fluorescence spectroscopy.
Delivery of undamaged quantum dots to the cell cytoplasm has been a challenge with existing techniques. Vector-based methods have resulted in aggregation and endosomal sequestration of quantum dots while electroporation can damage the semi-conducting particles and aggregate delivered dots in the cytosol. Via
Photovoltaic devices

The tunable absorption spectrum and high extinction coefficients of quantum dots make them attractive for light harvesting technologies such as photovoltaics. Quantum dots may be able to increase the efficiency and reduce the cost of today's typical silicon
Colloidal quantum dot photovoltaics would theoretically be cheaper to manufacture, as they can be made using simple chemical reactions.
Quantum dot only solar cells
Aromatic
Quantum dot in hybrid solar cells
Colloidal quantum dots are also used in inorganic–organic hybrid solar cells. These solar cells are attractive because of the potential for low-cost fabrication and relatively high efficiency.[101] Incorporation of metal oxides, such as ZnO, TiO2, and Nb2O5 nanomaterials into organic photovoltaics have been commercialized using full roll-to-roll processing.[101] A 13.2% power conversion efficiency is claimed in Si nanowire/PEDOT:PSS hybrid solar cells.[102]
Quantum dot with nanowire in solar cells
Another potential use involves capped single-crystal ZnO nanowires with CdSe quantum dots, immersed in mercaptopropionic acid as hole transport medium in order to obtain a QD-sensitized solar cell. The morphology of the nanowires allowed the electrons to have a direct pathway to the photoanode. This form of solar cell exhibits 50–60% internal quantum efficiencies.[103]
Nanowires with quantum dot coatings on silicon nanowires (SiNW) and carbon quantum dots. The use of SiNWs instead of planar silicon enhances the antiflection properties of Si.[104] The SiNW exhibits a light-trapping effect due to light trapping in the SiNW. This use of SiNWs in conjunction with carbon quantum dots resulted in a solar cell that reached 9.10% PCE.[104]
Graphene quantum dots have also been blended with organic electronic materials to improve efficiency and lower cost in photovoltaic devices and organic light emitting diodes (OLEDs) compared to graphene sheets. These graphene quantum dots were functionalized with organic ligands that experience photoluminescence from UV–visible absorption.[105]
Light-emitting diodes
Several methods are proposed for using quantum dots to improve existing
Quantum dot displays

Quantum dots are valued for displays because they emit light in very specific
A conventional color
Another method by which quantum dot displays can be achieved is the electroluminescent (EL) or electro-emissive method. This involves embedding quantum dots in each individual pixel. These are then activated and controlled via an electric current application.[108] Since this is often light emitting itself, the achievable colors may be limited in this method.[109] Electro-emissive QD-LED TVs exist in laboratories only.
The ability of QDs to precisely convert and tune a spectrum makes them attractive for LCD displays. Previous LCD displays can waste energy converting red-green poor, blue-yellow rich white light into a more balanced lighting. By using QDs, only the necessary colors for ideal images are contained in the screen. The result is a screen that is brighter, clearer, and more energy-efficient. The first commercial application of quantum dots was the Sony XBR X900A series of flat panel televisions released in 2013.[110]
In June 2006, QD Vision announced technical success in making a proof-of-concept
Photodetector devices
Quantum dot photodetectors (QDPs) can be fabricated either via solution-processing,
Photocatalysts
Quantum dots also function as photocatalysts for the light driven chemical conversion of water into hydrogen as a pathway to
Theory
Quantum dots are theoretically described as a point-like, or zero dimensional (0D) entity. Most of their properties depend on the dimensions, shape, and materials of which QDs are made. Generally, QDs present different thermodynamic properties from their bulk materials. One of these effects is melting-point depression. Optical properties of spherical metallic QDs are well described by the Mie scattering theory.
Quantum confinement in semiconductors

The energy levels of a single particle in a quantum dot can be predicted using the particle in a box model in which the energies of states depend on the length of the box. For an exciton inside a quantum dot, there is also the Coulomb interaction between the negatively charged electron and the positively charged hole. By comparing the quantum dot's size to the exciton Bohr radius, three regimes can be defined. In the 'strong confinement regime', the quantum dot's radius is much smaller than the exciton Bohr radius, respectively the confinement energy dominates over the Coulomb interaction.[116] In the 'weak confinement' regime, the quantum dot is larger than the exciton Bohr radius, respectively the confinement energy is smaller than the Coulomb interactions between electron and hole. The regime where the exciton Bohr radius and confinement potential are comparable is called the 'intermediate confinement regime'.[117]

- Band gap energy
- The band gap can become smaller in the strong confinement regime as the energy levels split up. The exciton Bohr radius can be expressed as:
- where aB = 0.053 nm is the Bohr radius, m is the mass, μ is the reduced mass, and εr is the size-dependent dielectric constant (relative permittivity). This results in the increase in the total emission energy (the sum of the energy levels in the smaller band gaps in the strong confinement regime is larger than the energy levels in the band gaps of the original levels in the weak confinement regime) and the emission at various wavelengths. If the size distribution of QDs is not enough peaked, the convolution of multiple emission wavelengths is observed as a continuous spectra.
- Confinement energy
- The exciton entity can be modeled using the particle in the box. The electron and the hole can be seen as hydrogen in the Bohr model with the hydrogen nucleus replaced by the hole of positive charge and negative electron mass. Then the energy levels of the exciton can be represented as the solution to the particle in a box at the ground level (n = 1) with the mass replaced by the reduced mass. Thus by varying the size of the quantum dot, the confinement energy of the exciton can be controlled.
- Bound exciton energy
- There is Coulomb attraction between the negatively charged electron and the positively charged hole. The negative energy involved in the attraction is proportional to Rydberg's energy and inversely proportional to square of the size-dependent dielectric constant[118] of the semiconductor. When the size of the semiconductor crystal is smaller than the exciton Bohr radius, the Coulomb interaction must be modified to fit the situation.
Therefore, the sum of these energies can be represented by Brus equation:
where μ is the reduced mass, a is the radius of the quantum dot, me is the free electron mass, mh is the hole mass, and εr is the size-dependent dielectric constant.
Although the above equations were derived using simplifying assumptions, they imply that the electronic transitions of the quantum dots will depend on their size. These quantum confinement effects are apparent only below the critical size. Larger particles do not exhibit this effect. This effect of quantum confinement on the quantum dots has been repeatedly verified experimentally[119] and is a key feature of many emerging electronic structures.[120]
The Coulomb interaction between confined carriers can also be studied by numerical means when results unconstrained by asymptotic approximations are pursued.[121]
Besides confinement in all three dimensions (that is, a quantum dot), other quantum confined semiconductors include:
- Quantum wires, which confine electrons or holes in two spatial dimensions and allow free propagation in the third.
- Quantum wells, which confine electrons or holes in one dimension and allow free propagation in two dimensions.
Models
A variety of theoretical frameworks exist to model optical, electronic, and structural properties of quantum dots. These may be broadly divided into quantum mechanical, semiclassical, and classical.
Quantum mechanics
Quantum mechanical models and simulations of quantum dots often involve the interaction of electrons with a pseudopotential or random matrix.[122]
Semiclassical
Semiclassical models of quantum dots frequently incorporate a chemical potential. For example, the thermodynamic chemical potential of an N-particle system is given by
whose energy terms may be obtained as solutions of the Schrödinger equation. The definition of capacitance,
with the potential difference
may be applied to a quantum dot with the addition or removal of individual electrons,
Then
is the quantum capacitance of a quantum dot, where we denoted by I(N) the ionization potential and by A(N) the electron affinity of the N-particle system.[123]
Classical mechanics
Classical models of electrostatic properties of electrons in quantum dots are similar in nature to the Thomson problem of optimally distributing electrons on a unit sphere.
The classical electrostatic treatment of electrons confined to spherical quantum dots is similar to their treatment in the Thomson,[124] or plum pudding model, of the atom.[125]
The classical treatment of both two-dimensional and three-dimensional quantum dots exhibit electron shell-filling behavior. A "periodic table of classical artificial atoms" has been described for two-dimensional quantum dots.[126] As well, several connections have been reported between the three-dimensional Thomson problem and electron shell-filling patterns found in naturally occurring atoms found throughout the periodic table.[127] This latter work originated in classical electrostatic modeling of electrons in a spherical quantum dot represented by an ideal dielectric sphere.[128]
History
For thousands of years, glassmakers were able to make colored glass by adding different dusts and powdered elements such as silver, gold and cadmium and then played with different temperatures to produce shades of glass. In the 19th century, scientists started to understand how glass color depended on elements and heating-cooling techniques. It was also found that for the same element and preparation, the color depended on the dust particles' size.[129][130]
Herbert Fröhlich in the 1930s first explored the idea that material properties can depend on the macroscopic dimensions of a small particle due to quantum size effects.[131]
The first quantum dots were synthesized in a glass matrix by Alexei A. Onushchenko and Alexey Ekimov in 1981 at the Vavilov State Optical Institute[132][133][134][135] and independently in colloidal suspension[136] by Louis E. Brus team at Bell Labs in 1983.[137][138] They were first theorized by Alexander Efros in 1982.[139] It was quickly identified that the optical changes that appeared for very small particles were due to quantum mechanical effects.[129]
The term quantum dot first appeared in a paper first authored by Mark Reed in 1986.[140] According to Brus, the term "quantum dot" was coined by Daniel S. Chemla while they were working at Bell Labs.[141]
In 1993, David J. Norris, Christopher B. Murray and Moungi Bawendi at the Massachusetts Institute of Technology reported on a hot-injection synthesis method for producing reproducible quantum dots with well-defined size and with high optical quality. The method opened the door to the development of large-scale technological applications of quantum dots in a wide range of areas.[142][129]
The Nobel Prize in Chemistry 2023 was awarded to Moungi Bawendi, Louis E. Brus and Alexey Ekimov "for the discovery and synthesis of quantum dots."[143]
See also
References
- PMID 37947733.
- ^ Silbey, Robert J.; Alberty, Robert A.; Bawendi, Moungi G. (2005). Physical Chemistry (4th ed.). John Wiley & Sons. p. 835.
- S2CID 4367436.
- doi:10.1063/1.881393.
- S2CID 4424927.
- PMID 31844043.
- S2CID 235215237.
- PMID 37236922.
- ^ .
- ^ Brus, L. E. (2007). "Chemistry and Physics of Semiconductor Nanocrystals" (PDF). Retrieved 7 July 2009.
- ^ "Quantum Dots". Nanosys – Quantum Dot Pioneers. Retrieved 4 December 2015.
- ISSN 0003-6951.
- S2CID 118664135.
- PMID 21806165.
- PMID 29109549.
- ISSN 1050-2947.
- ^ PMID 15681376.
- S2CID 1720328.
- S2CID 41348562.
- S2CID 94993172.
- .
- S2CID 139004694.
- S2CID 97571829.
- ^ PMID 36599825.
- ISSN 2076-3417.
- PMID 31763517.
- ^ ISSN 0925-8388.
- ^ ISSN 1876-6102.
- ^ ISSN 0379-6779.
- S2CID 207060827.
- PMID 25633588.
- PMID 15826104.
- S2CID 2461258.
- PMID 15755110.
- S2CID 121602427.
- S2CID 12178919.
- S2CID 121329624.
- S2CID 118926523.
- S2CID 123881981.
- S2CID 95855020.
- S2CID 95841139.
- S2CID 9107033.
- PMID 28530219.
- PMID 28443889.
- S2CID 93219029.
- PMID 9975303.
- S2CID 119036164.
- S2CID 124809958.
- S2CID 28558725.
- S2CID 4429190.
- PMID 23441602.
- ^ Soutter, Will (30 May 2013). "Continuous Flow Synthesis Method for Fluorescent Quantum Dots". AZo Nano. Retrieved 19 July 2015.
- ^ Quantum Materials Corporation and the Access2Flow Consortium (2011). "Quantum materials corp achieves milestone in High Volume Production of Quantum Dots". Archived from the original on 10 February 2015. Retrieved 7 July 2011.
{{cite news}}: CS1 maint: numeric names: authors list (link) - ^ "Nanoco and Dow tune in for sharpest picture yet". The Times. 25 September 2014. Retrieved 9 May 2015.
- ^ MFTTech (24 March 2015). "LG Electronics Partners with Dow to Commercialize LGs New Ultra HD TV with Quantum Dot Technology". Archived from the original on 18 May 2015. Retrieved 9 May 2015.
- S2CID 205060500.
- ^ PMID 16451849.
- ^ PMID 19684286.
- ^ PMID 22853558.
- PMID 28890669.
- PMID 21980346.
- S2CID 54915101.
- PMID 15614393.
- S2CID 7125377.
- PMID 17891134.
- S2CID 205236024.
- S2CID 4812108. Archived from the original(PDF) on 2 May 2019. Retrieved 16 September 2007.
- .
- S2CID 45625439.
- S2CID 13152124.
- PMID 30052199.
- PMID 20922257.
- ISSN 0959-9428.
- S2CID 4400136.
- PMID 30530939.
- PMID 26798011.
- ^ PMID 19333416.
- ISBN 978-1-68108-519-7. Archived from the originalon 14 May 2019. Retrieved 24 December 2017.
- PMID 22458433.
- PMID 25157589.
- .
- PMID 15731014.
- S2CID 30071440.
- PMID 18425138.
- PMID 12235356.
- PMID 24122039.
- PMID 15541352.
- S2CID 206556385.
- ^ PMID 14733586.
- PMID 18419147.
- PMID 28278581.
- S2CID 9007994.
- S2CID 20341752.
- .
- PMID 17891134.
- PMID 23341631.
- S2CID 4186651.
- S2CID 226335202.
- PMID 34785654.
- ^ PMID 26509283.
- ^ PMID 20648282.
- PMID 26174964.
- PMID 17503867.
- ^ PMID 24665986.
- PMID 21650464.
- ^ "Nano LEDs printed on silicon". nanotechweb.org. 3 July 2009. Archived from the original on 26 September 2017.
- ^ "Quantum Dots: Solution for a Wider Color Gamut". pid.samsungdisplay.com. Retrieved 1 November 2018.
- ^ "A Guide to the Evolution of Quantum Dot Displays". pid.samsungdisplay.com. Retrieved 1 November 2018.
- ^ "Quantum dot white and colored light emitting diodes". patents.google.com. Retrieved 1 November 2018.
- ^ Bullis, Kevin (11 January 2013). "Quantum Dots Produce More Colorful Sony TVs". MIT Technology Review. Retrieved 19 July 2015.
- S2CID 4016378.
- S2CID 7684370.
- .
- ^ Palomaki, P.; Keuleyan, S. (25 February 2020). "Move over CMOS, here come snapshots by quantum dots". IEEE Spectrum. Retrieved 20 March 2020.
- PMID 23590186.
- ISSN 0022-2313.
- S2CID 22966827.
- ^ Brandrup, J.; Immergut, E.H. (1966). Polymer Handbook (2 ed.). New York: Wiley. pp. 240–246.
- PMID 21952415.
- ^ Greenemeier, L. (5 February 2008). "New Electronics Promise Wireless at Warp Speed". Scientific American.
- .
- S2CID 9344722.
- ^
Iafrate, G. J.; Hess, K.; Krieger, J. B.; Macucci, M. (1995). "Capacitive nature of atomic-sized structures". Physical Review B. 52 (15): 10737–10739. PMID 9980157.
- ^ .
- ^ Bednarek, S.; Szafran, B.; Adamowski, J. (1999). "Many-electron artificial atoms". Physical Review B. 59 (20): 13036–13042. .
- ^
Bedanov, V. M.; Peeters (1994). "Ordering and phase transitions of charged particles in a classical finite two-dimensional system". Physical Review B. 49 (4): 2667–2676. PMID 10011100.
- S2CID 118480104.
- S2CID 118480104.
- ^ a b c Linke, Heiner (3 October 2023). "Quantum dots — seeds of nanoscience" (PDF). The Royal Swedish Academy of Sciences.
- PMID 35325541.
- ^ Robinson2023-10-11T17:50:00+01:00, Julia. "The quantum dot story". Chemistry World. Retrieved 20 October 2023.
{{cite web}}: CS1 maint: numeric names: authors list (link) - ^ Ekimov, A. I.; Onushchenko, A. A. (1981). "Квантовый размерный эффект в трехмерных микрокристаллах полупроводников" [The quantum size effect in three-dimensional semiconductor microcrystals] (PDF). JETP Letters (in Russian). 34: 363–366.
- ^ Ekimov, A. I.; Onushchenko, A. A. (1982). "Quantum size effect in the optical-spectra of semiconductor micro-crystals". Soviet Physics Semiconductors-USSR. 16 (7): 775–778.
- .
- ^ "Nanotechnology Timeline". National Nanotechnology Initiative.
- ^ Kolobkova, E. V.; Nikonorov, N. V.; Aseev, V. A. (2012). "Optical Technologies Silver Nanoclusters Influence on Formation of Quantum Dots in Fluorine Phosphate Glasses". Scientific and Technical Journal of Information Technologies, Mechanics and Optics. 5 (12).
- ISSN 0021-9606.
- S2CID 54779723.
- ^ "History of Quantum Dots". Nexdot. Retrieved 8 October 2020.
- ISSN 0734-211X.
- ^ "Louis E. Brus life story". www.kavliprize.org. Retrieved 4 October 2023.
- ^ Palma, Jasmine; Wang, Austin H. (6 October 2023). "One Small Quantum Dot, One Giant Leap for Nanoscience: Moungi Bawendi '82 Wins Nobel Prize in Chemistry". The Harvard Crimson.
- ^ "The Nobel Prize in Chemistry 2023". NobelPrize.org. Retrieved 6 October 2023.
Further reading
- Delerue, C.; Lannoo, M. (2004). Nanostructures: Theory and Modelling. Springer. p. 47. epitaxial techniques), nanocrystalsby gas-phase, liquid-phase, and solid-phase approaches.
- Norris, D. J. (1995). "Measurement and Assignment of the Size-Dependent Optical Spectrum in Cadmium Selenide (CdSe) Quantum Dots, PhD thesis, MIT". of a QD vs. particle diameter.
External links
- Quantum Dots: Technical Status and Market Prospects
- Quantum dots that produce white light could be the light bulb's successor
- Single quantum dots optical properties
- Quantum dot on arxiv.org
- Quantum Dots Research and Technical Data
- Simulation and interactive visualization of Quantum Dots wave function


![{\displaystyle {\begin{aligned}E_{\textrm {confinement}}&={\frac {\hbar ^{2}\pi ^{2}}{2a^{2}}}\left({\frac {1}{m_{\rm {e}}}}+{\frac {1}{m_{\rm {h}}}}\right)={\frac {\hbar ^{2}\pi ^{2}}{2\mu a^{2}}}\\[6px]E_{\textrm {exciton}}&=-{\frac {1}{\varepsilon _{\rm {r}}^{2}}}{\frac {\mu }{m_{\rm {e}}}}R_{y}=-R_{y}^{*}\\[6px]E&=E_{\textrm {bandgap}}+E_{\textrm {confinement}}+E_{\textrm {exciton}}\\&=E_{\textrm {bandgap}}+{\frac {\hbar ^{2}\pi ^{2}}{2\mu a^{2}}}-R_{y}^{*}\end{aligned}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/f097b44c2c3e15f62d862f1a77b6de08017e042f)




