Quorum sensing
This article needs additional citations for verification. (April 2024) |
In
In addition to its function in biological systems, quorum sensing has several useful applications for computing and robotics. In general, quorum sensing can function as a decision-making process in any
Discovery
The first observations of an autoinducer-controlled phenotype in bacteria were reported in 1970, by Kenneth Nealson, Terry Platt, and
Dr. Stephen Winnans did not believe the word "autoinducer" fully enveloped the true process, so introduced the world to the term "Quorum Sensing" in 1994 through his article review of autoinduction in bacteria. This new phrase helped relieve confusion between 'autoinduction" and "autoregulation". The phrase "Quorum Sensing" was not stumbled onto, but rather created through trial and error. Dr. Winnans created many options, such as "gridlockins", "communiolins", and "quoromones".[10] Eventually, it was "Quorum Sensing that stuck, and Dr. Stephen Winnans phrase spread quickly into the world. Quorum Sensing is now involved in practically every article written about autoinduction throughout the scientific community. [11]
Bacteria
| Part of a series on |
| Microbial and microbot movement |
|---|
 |
| Microswimmers |
| Molecular motors |

Some of the best-known examples of quorum sensing come from studies of bacteria. Bacteria use quorum sensing to regulate certain phenotype expressions, which in turn, coordinate their behavio rs. Some common phenotypes include biofilm formation, virulence factor expression, and motility. Certain bacteria are able to use quorum sensing to regulate bioluminescence, nitrogen fixation and sporulation.[12]
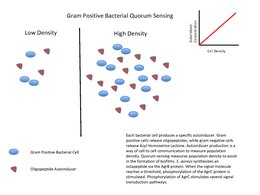
The quorum-sensing function is based on the local density of the bacterial population in the immediate environment.[13] It can occur within a single bacterial species, as well as between diverse species. Both gram-positive and gram-negative bacteria use quorum sensing, but there are some major differences in their mechanisms.[14]
Mechanism
For the bacteria to use quorum sensing constitutively, they must possess three abilities: secretion of a signaling molecule, secretion of an autoinducer (to detect the change in concentration of signaling molecules), and regulation of gene transcription as a response.[12] This process is highly dependent on the diffusion mechanism of the signaling molecules. QS signaling molecules are usually secreted at a low level by individual bacteria. At low cell density, the molecules may just diffuse away. At high cell density, the local concentration of signaling molecules may exceed its threshold level, and trigger changes in gene expression.[14]
Gram-positive bacteria
Gram-positive bacteria use autoinducing peptides (AIP) as their autoinducers.[15]
When gram-positive bacteria detect high concentration of AIPs in their environment, that happens by way of AIPs binding to a receptor to activate a kinase. The kinase phosphorylates a transcription factor, which regulates gene transcription. This is called a two-component system.
Another possible mechanism is that AIP is transported into the cytosol, and binds directly to a transcription factor to initiate or inhibit transcription.[15]
Gram-negative bacteria
Gram-negative bacteria produce N-acyl homoserine lactones (AHL) as their signaling molecule.[15] Usually AHLs do not need additional processing, and bind directly to transcription factors to regulate gene expression.[14]
Some gram-negative bacteria may use the two-component system as well.[15]
Examples
Aliivibrio fischeri
The bioluminescent bacterium
Curvibacter sp.
Curvibacter sp. is a gram-negative curved rod-formed bacterium which is the main colonizer of the epithelial cell surfaces of the early branching metazoan Hydra vulgaris.[16][17] Sequencing the complete genome uncovered a circular chromosome (4.37 Mb), a plasmid (16.5 kb), and two operons coding each for an AHL (N-acyl-homoserine lactone) synthase (curI1 and curI2) and an AHL receptor (curR1 and curR2).[17] Moreover, a study showed that these host associated Curvibacter bacteria produce a broad spectrum of AHL, explaining the presence of those operons.[17] As mentioned before, AHL are the quorum sensing molecules of gram-negative bacteria, which means Curvibacter has a quorum sensing activity.
Even though their function in host-microbe interaction is largely unknown, Curvibacter quorum-sensing signals are relevant for host-microbe interactions.[17] Indeed, due to the oxidoreductase activity of Hydra, there is a modification of AHL signalling molecules (3-oxo-homoserine lactone into 3-hydroxy-homoserine lactone) which leads to a different host-microbe interaction. On one hand, a phenotypic switch of the colonizer Curvibacter takes place. The most likely explanation is that the binding of 3-oxo-HSL and 3-hydroxy-HSL causes different conformational changes in the AHL receptors curR1 and curR2. As a result, there is a different DNA-binding motif affinity and thereby different target genes are activated.[17] On the other hand, this switch modifies its ability to colonize the epithelial cell surfaces of Hydra vulgaris.[17] Indeed, one explanation is that with a 3-oxo-HSL quorum-sensing signal, there is an up-regulation of flagellar assembly. Yet, flagellin, the main protein component of flagella, can act as an immunomodulator and activate the innate immune response in Hydra. Therefore, bacteria have less chance to evade the immune system and to colonize host tissues.[17] Another explanation is that 3-hydroxy-HSL induces carbon metabolism and fatty acid degradation genes in Hydra. This allows the bacterial metabolism to adjust itself to the host growth conditions, which is essential for the colonization of the ectodermal mucus layer of Hydrae.[17]
Enterococcus faecalis
Enterococcus faecalis is an opportunistic, Gram-positive bacteria that forms biofilm in glass. This process is also known as forming a biofilm in vitro. The presence of (Esp), a certain cell surface protein, aids the formation of a biofilm by Enterococcus faecalis.[18]
Escherichia coli
In the gram-negative bacterium
E. coli and Salmonella enterica do not produce AHL signals commonly found in other gram-negative bacteria. However, they have a receptor that detects AHLs from other bacteria and change their gene expression in accordance with the presence of other "quorate" populations of gram-negative bacteria.[19] AHL quorum sensing regulates a wide range of genes through cell density. Other species of bacteria produce AHLs that Eschericia and Salmonella can detect. E.coli and Salmonella produce a receptor like protein (SdiA) allowing the amino acid sequence that is similar to AHL show AHLs can be found in the bovine rumen and E. Coli responds to AHLs taken out of the bovine rumen. Most animals do not have AHL in their gastrointestinal tracts. [20] .
Salmonella enterica
Staphylococcus aureus
Staphylococcus aureus is a type of pathogen that causes infection to the skin and soft tissue and can lead to a variety of more severe diseases such as osteomyelitis, pneumonia, and endocarditis. Staphylococcus aureus uses biofilms in order to increase its chances of survival by becoming resistant to antibiotics. Biofilms help Staphylococcus aureus become up to 1500 times more resistant to antibiofilm agents which try to break down biofilms formed by Staphylococcus aureus.[26]
Pseudomonas aeruginosa
The environmental bacterium and opportunistic pathogen
Another form of
Acinetobacter sp.
It has recently been found that Acinetobacter sp. also show quorum sensing activity. This bacterium, an emerging pathogen, produces AHLs.[31] Acinetobacter sp. shows both quorum sensing and quorum quenching activity. It produces AHLs and can also degrade the AHL molecules.[31]
Aeromonas sp.
This bacterium was previously considered a fish pathogen, but it has recently emerged as a human pathogen.[32] Aeromonas sp. have been isolated from various infected sites from patients (bile, blood, peritoneal fluid, pus, stool and urine). All isolates produced the two principal AHLs, N-butanoylhomoserine lactone (C4-HSL) and N-hexanoyl homoserine lactone (C6-HSL). It has been documented that Aeromonas sobria has produced C6-HSL and two additional AHLs with N-acyl side chain longer than C6.[33]
Yersinia
The YenR and YenI proteins produced by the
Molecules involved
Three-dimensional structures of proteins involved in quorum sensing were first published in 2001, when the
A database of quorum-sensing peptides is available under the name Quorumpeps.[41][42]
Certain bacteria can produce enzymes called lactonases that can target and inactivate AHLs. Researchers have developed novel molecules which block the signalling receptors of bacteria ("Quorum quenching"). mBTL is a compound that has been shown to inhibit quorum sensing and decrease the amount of cell death by a significant amount.[43] Additionally, researchers are also examining the role of natural compounds (such as caffeine) as potential quorum sensing inhibitors.[44] Research in this area has been promising and could lead to the development of natural compounds as effective therapeutics.
Evolution
Sequence analysis
The majority of quorum sensing systems that fall under the "two-gene" (an autoinducer synthase coupled with a receptor molecule) paradigm as defined by the
Although examples of horizontal gene transfer are apparent in LuxI, LuxR, and LuxS phylogenies, they are relatively rare. This result is in line with the observation that quorum sensing genes tend to control the expression of a wide array of genes scattered throughout the bacterial chromosome. A recent acquisition by horizontal gene transfer would be unlikely to have integrated itself to this degree. Given that the majority of autoinducer–synthase/receptor pairs occur in tandem in bacterial genomes, it is also rare that they switch partners and so pairs tend to co-evolve.[46]
In quorum sensing genes of Gammaproteobacteria, which includes Pseudomonas aeruginosa and Escherichia coli, the LuxI/LuxR genes form a functional pair, with LuxI as the auto-inducer synthase and LuxR as the receptor. Gammaproteobacteria are unique in possessing quorum sensing genes, which, although functionally similar to the LuxI/LuxR genes, have a markedly divergent sequence.[46] This family of quorum-sensing homologs may have arisen in the Gammaproteobacteria ancestor, although the cause of their extreme sequence divergence yet maintenance of functional similarity has yet to be explained. In addition, species that employ multiple discrete quorum sensing systems are almost all members of the Gammaproteobacteria, and evidence of horizontal transfer of quorum sensing genes is most evident in this class.[45][46]
Interaction of quorum-sensing molecules with mammalian cells and its medical applications
Next to the potential antimicrobial functionality, quorum-sensing derived molecules, especially the peptides, are being investigated for their use in other therapeutic domains as well, including immunology, central nervous system disorders and oncology. Quorum-sensing peptides have been demonstrated to interact with cancer cells, as well as to permeate the blood–brain barrier reaching the brain parenchyma.[47][48][49]
Role of quorum sensing in biofilm development
Quorum sensing is used by bacteria to form biofilms. Quorum sensing is used by bacteria to form biofilms because the process determines if the minimum number of bacteria necessary for biofilm formation are present. The criteria to form a biofilm is dependent on a certain density of bacteria rather than a certain number of bacteria being present. When aggregated in high enough densities, some bacteria may form biofilms to protect themselves from biotic or abiotic threats.[50] Quorum sensing is used by both Gram-positive and Gram-negative bacteria because it aids cellular reproduction. Once in a biofilm, bacteria can communicate with other bacteria of the same species. Bacteria can also communicate with other species of bacteria. This communication is enabled through autoinducers used by the bacteria.[51]
Additionally, certain responses can be generated by the host organism in response to the certain bacterial autoinducers. Despite the fact that specific bacterial quorum sensing systems are different, for example the target genes, signal relay mechanisms, and chemical signals used between bacteria, the ability to coordinate gene expression for a specific species of bacteria remains the same. This ability alludes to the larger idea that bacteria have potential to become a multicellular bacterial body.[52]
Secondly, biofilms may also serve to transport nutrients into the microbial community or transport toxins out by means of channels that permeate the extracellular polymeric matrix (like cellulose) that holds the cells together. Finally, biofilms are an ideal environment for horizontal gene transfer through either conjugation or environmental DNA (eDNA) that exists in the biofilm matrix.[50]
The process of biofilm development is often triggered by environmental signals, and bacteria are proven to require flagella to successfully approach a surface, adhere to it, and form the biofilm.[50] As cells either replicate or aggregate in a location, the concentration of autoinducers outside of the cells increases until a critical mass threshold is reached. At this point, it is energetically unfavorable for intracellular autoinducers to leave the cell and they bind to receptors and trigger a signaling cascade to initiate gene expression and begin secreting an extracellular polysaccharide to encase themselves inside.[53]
Archaea
Examples
Methanosaeta harundinacea 6Ac
Viruses
A mechanism involving
Plants
QS is important to plant-pathogen interactions, and their study has also contributed to the QS field more generally.
The role of AHLs having long carbon-chains (C12, C14), which have an unknown receptor mechanism, is less well understood than AHLs having short carbon-chains (C4, C6, C8), which are perceived by the G protein-coupled receptor. A phenomenon called "AHL priming", which is a dependent signalling pathway, enhanced our knowledge of long-chain AHLs. The role of quorum-sensing molecules was better explained according to three categories: host physiology–based impact of quorum sensing molecules; ecological effects; and cellular signaling. Calcium signalling and calmodulin have a large role in short-chain AHLs' response in Arabidopsis. Research was also conducted on barley and the crop called yam bean (Pachyrhizus erosus) that reveals the AHLs determining the detoxification enzymes called GST were found less in yam bean.[63]
Quorum sensing-based regulatory systems are necessary to plant-disease-causing bacteria. Looking towards developing new strategies based on plant-associated microbiomes, the aim of further study is to improve the quantity and quality of the food supply. Further research into this inter-kingdom communication also enhances the possibility of learning about quorum sensing in humans.[64]This exploration could open new avenues for managing microbial communities in agricultural settings, potentially leading to the development of more sustainable farming practices that leverage natural microbial processes to boost crop resilience and productivity.
Quorum quenching
Quorum quenching is the process of preventing quorum sensing by disrupting signalling.[65] This is achieved by inactivating signalling enzymes, by introducing molecules that mimic signalling molecules and block their receptors, by degrading signalling molecules themselves, or by a modification of the quorum sensing signals due to an enzyme activity.[17][65][66][67]
Inhibition
Closantel and triclosan are known inhibitors of quorum sensing enzymes.[68] Closantel induces aggregation of the histidine kinase sensor in two-component signalling. The latter disrupts the synthesis of a class of signalling molecules known as N-acyl homoserine lactones (AHLs) by blocking the enoyl-acyl carrier protein (ACP) reductase.[68][69]
Mimicry
Two groups of well-known mimicking molecules include halogenated furanones, which mimic AHL molecules, and synthetic Al peptides (AIPs), which mimic naturally occurring AIPs. These groups inhibit receptors from binding substrate or decrease the concentration of receptors in the cell.[68] Furanones have also been found to act on AHL-dependant transcriptional activity, whereby the half life of the autoinducer-binding LuxR protein is significantly shortened.[70]
Degradation
Recently, a well-studied quorum quenching bacterial strain (KM1S) was isolated and its AHL degradation kinetics were studied using rapid resolution liquid chromatography (RRLC).[71] RRLC efficiently separates components of a mixture to a high degree of sensitivity, based on their affinities for different liquid phases.[72] It was found that the genome of this strain encoded an inactivation enzyme with distinct motifs targeting the degradation of AHLs.[71]
Modifications
As mentioned before, N-acyl-homoserine lactones (AHL) are the quorum sensing signaling molecules of the gram-negative bacteria. However, these molecules may have different functional groups on their acyl chain, and also a different length of acyl chain. Therefore, there exist many different AHL signaling molecules, for example, 3-oxododecanoyl-L-homoserine lactone (3OC12-HSL) or 3-hydroxydodecanoyl-L-homoserine lactone (3OHC12-HSL). The modification of those quorum sensing (QS) signaling molecules is another sort of quorum quenching. This can be carried out by an oxidoreductase activity.[17] As an example, we will discuss the interaction between a host, Hydra vulgaris, and the main colonizer of its epithelial cell surfaces, Curvibacter spp. Those bacteria produce 3-oxo-HSL quorum sensing molecules.[17] However, the oxidoreductase activity of the polyp Hydra is able to modify the 3-oxo-HSL into their 3-hydroxy-HSL counterparts.[17] We can characterize this as quorum quenching since there is an interference with quorum sensing molecules. In this case, the outcomes differ from simple QS inactivation: the host modification results in a phenotypic switch of Curvibacter, which modifies its ability to colonize the epithelial cell surfaces of H. vulgaris.[17]
Applications
Applications of quorum quenching that have been exploited by humans include the use of AHL-degrading bacteria in aquacultures to limit the spread of diseases in aquatic populations of fish, mollusks and crustaceans.[73] This technique has also been translated to agriculture, to restrict the spread of pathogenic bacteria that use quorum sensing in plants.[73][74] Anti-biofouling is another process that exploits quorum quenching bacteria to mediate the dissociation of unwanted biofilms aggregating on wet surfaces, such as medical devices, transportation infrastructure and water systems.[73][75] Quorum quenching is recently studied for the control of fouling and emerging contaminants in electro membrane bioreactors (eMBRs) for the advanced treatment of wastewater.[76] Extracts of several traditional medicinal herbs display quorum quenching acivity, and have potential antibacterial applications.[77][78]
Social insects
Social insect colonies are an excellent example of a
Examples
Ants
Colonies of the ant Temnothorax albipennis nest in small crevices between rocks. When the rocks shift and the nest is broken up, these ants must quickly choose a new nest to move into. During the first phase of the decision-making process, a small portion of the workers leave the destroyed nest and search for new crevices. When one of these scout ants finds a potential nest, she assesses the quality of the crevice based on a variety of factors including the size of the interior, the number of openings (based on light level), and the presence or absence of dead ants.[79][80] The worker then returns to the destroyed nest, where she waits for a short period before recruiting other workers to follow her to the nest that she has found, using a process called tandem running. The waiting period is inversely related to the quality of the site; for instance, a worker that has found a poor site will wait longer than a worker that encountered a good site.[81] As the new recruits visit the potential nest site and make their own assessment of its quality, the number of ants visiting the crevice increases. During this stage, ants may be visiting many different potential nests. However, because of the differences in the waiting period, the number of ants in the best nest will tend to increase at the greatest rate. Eventually, the ants in this nest will sense that the rate at which they encounter other ants has exceeded a particular threshold, indicating that the quorum number has been reached.[82] Once the ants sense a quorum, they return to the destroyed nest and begin rapidly carrying the brood, queen, and fellow workers to the new nest. Scouts that are still tandem-running to other potential sites are also recruited to the new nest, and the entire colony moves. Thus, although no single worker may have visited and compared all of the available options, quorum sensing enables the colony as a whole to quickly make good decisions about where to move.
Honey bees
The quorum sensing process in honey bees is similar to the method used by Temnothorax ants in several ways. A small portion of the workers leave the swarm to search out new nest sites, and each worker assesses the quality of the cavity it finds. The worker then returns to the swarm and recruits other workers to her cavity using the honey bee waggle dance. However, instead of using a time delay, the number of dance repetitions the worker performs is dependent on the quality of the site. Workers that found poor nests stop dancing sooner, and can, therefore, be recruited to the better sites. Once the visitors to a new site sense that a quorum number (usually 10–20 bees) has been reached, they return to the swarm and begin using a new recruitment method called piping. This vibration signal causes the swarm to take off and fly to the new nest location. In an experimental test, this decision-making process enabled honey bee swarms to choose the best nest site in four out of five trials.[83][84]
Synthetic biology
Quorum sensing has been engineered using
Computing and robotics
Quorum sensing can be a useful tool for improving the function of self-organizing networks such as the SECOAS (Self-Organizing Collegiate Sensor) environmental monitoring system. In this system, individual nodes sense that there is a population of other nodes with similar data to report. The population then nominates just one node to report the data, resulting in power savings.[90] Ad hoc wireless networks can also benefit from quorum sensing, by allowing the system to detect and respond to network conditions.[91]
Quorum sensing can also be used to coordinate the behavior of autonomous robot swarms. Using a process similar to that used by Temnothorax ants, robots can make rapid group decisions without the direction of a controller.[92]
See also
References
- PMID 14507383.
- PMID 31572366.
- PMID 27510864.
- ^ PMID 36130487.
- PMID 28270076.
- ^ PMID 5473898.
- ^ S2CID 3926735.
- S2CID 34239721.
- PMID 8288518.
- ^ https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2391307/#:~:text=The%20term%20%E2%80%9Cquorum%20sensing%E2%80%9D%20was,right%20to%20the%20young%20professor.
- ^ https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2391307/#:~:text=The%20term%20%E2%80%9Cquorum%20sensing%E2%80%9D%20was,right%20to%20the%20young%20professor.
- ^ S2CID 30007165.
- S2CID 1099089.
- ^ PMID 10607620.
- ^ PMID 23125205.
- ^ "Curvibacter fontana sp. nov., a microaerobic bacteria isolated from well water". ResearchGate. Retrieved 2019-03-13.
- ^ PMID 28923926.
- ^ https://www.ncbi.nlm.nih.gov/pmc/articles/PMC365672/
- PMID 15130116.
- ^ https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3078957//
- PMID 11544237.
- PMID 9495757.
- PMID 12562806.
- PMID 18665275.
- PMID 19820103.
- ^ https://www.sciencedirect.com/science/article/pii/S2590207523000722#:~:text=At%20the%20beginning%20of%20biofilm,%2Danchored%20(CWA)%20proteins.
- PMID 11807075.
- PMID 16476569.
- ISBN 978-1-904455-19-6. Retrieved 1 May 2022.
- PMID 38357290.
- ^ PMID 21385437.
- PMID 22701365.
- S2CID 6761810.
- S2CID 3100775.
- ^ PMID 16452428.
- ^ PMID 21362062.
- PMID 11435117.
- S2CID 4334017. Archived from the original(PDF) on 2004-06-22. Retrieved 2004-04-28.
- PMID 15456522.
- PMID 11425711.
- PMID 23180797.
- S2CID 21031922.
- PMID 24143808.
- PMID 23598500.
- ^ PMID 11496014.
- ^ PMID 15014168.
- PMID 25780927.
- S2CID 28064836.
- PMID 26536593.
- ^ PMID 23903045.
- ^ https://pubmed.ncbi.nlm.nih.gov/11544353/
- ^ https://pubmed.ncbi.nlm.nih.gov/11544353/
- ^ "How Quorum Sensing Works". American Society for Microbiology. Retrieved 2023-04-21.
- PMID 22237544.
- S2CID 90839014.
- ^ Stokar-Avihail A, Tal N, Erez Z, Lopatina A, Sorek R. Widespread Utilization of Peptide Communication in Phages Infecting Soil and Pathogenic Bacteria. Cell host & microbe. 2019 May 8;25(5):746-55.
- PMID 28099413.
- OCLC 40075829.
- PMID 11931774.
- PMID 12198141.
- PMID 12087407.
- PMID 25914699.
- PMID 26347761.
- S2CID 3926735.
- ^ PMID 28446917.
- PMID 21385437.
- S2CID 28637950.
- ^ PMID 30894996.
- S2CID 39018931.
- PMID 11932456.
- ^ S2CID 12407347.
- PMID 17154122.
- ^ PMID 26432822.
- PMID 22771187.
- .
- S2CID 52134789.
- PMID 34094582.
- PMID 33194208.
- PMID 16555783.
- PMID 17148163.
- S2CID 15360262.
- .
- .
- .
- S2CID 4426454.
- S2CID 221510088.
- PMID 22215088.
- PMID 27437587.
- PMID 31511505.
- ^ Britton M, Sacks L (2004). "The SECOAS Project—Development of a Self-Organising, Wireless Sensor Network for Environmental Monitoring" (PDF). SANPA. Archived from the original (PDF) on 2008-12-16.
- ISBN 0-7803-8916-6.
- CiteSeerX 10.1.1.161.6407.
Further reading
- Dedicated issue of Philosophical Transactions B on quorum sensing (2007). Some articles are freely available.
- High citation review: Waters CM, Bassler BL (2005). "Quorum sensing: cell-to-cell communication in bacteria". Annual Review of Cell and Developmental Biology. 21: 319–346. PMID 16212498.
External links
- The Quorum Sensing Website
- Cell-to-Cell Communication in Bacteria Archived 2013-05-17 at the Wayback Machine
- The SECOAS project—Development of a Self-Organising, Wireless Sensor Network for Environmental Monitoring
- Measurement of Space: From Ants to Robots
- Instant insight into quorum sensing from the Royal Society of Chemistry
- Bonnie Bassler: Discovering bacteria's amazing communication system Archived 2011-12-05 at the Wayback Machine
- Bonnie Bassler's seminar: "Cell-Cell Communication in Bacteria"

