RNA world
The RNA world is a hypothetical stage in the
- Like DNA, RNA can store and replicate genetic information.
- Like protein
One of the most critical components of cells, the ribosome, is composed primarily of RNA. Ribonucleotide moieties in many coenzymes, such as acetyl-CoA, NADH, FADH, and F420, may be surviving remnants of covalently bound coenzymes in an RNA world.[11]
Although RNA is fragile, some ancient RNAs may have evolved the ability to
If the RNA world existed, it was probably followed by an age characterized by the evolution of
History
One of the challenges in studying abiogenesis is that the system of reproduction and metabolism utilized by all extant life involves three distinct types of interdependent macromolecules (DNA, RNA, and proteins). This suggests that life could not have arisen in its current form, which has led researchers to hypothesize mechanisms whereby the current system might have arisen from a simpler precursor system.[14] American molecular biologist Alexander Rich was the first to posit a coherent hypothesis on the origin of nucleotides as precursors of life.[15] In an article he contributed to a volume issued in honor of Nobel-laureate physiologist Albert Szent-Györgyi, he explained that the primitive Earth's environment could have produced RNA molecules (polynucleotide monomers) that eventually acquired enzymatic and self-replicating functions.[16]
Further concept of RNA as a primordial molecule can be found in papers by Francis Crick[17] and Leslie Orgel,[18] as well as in Carl Woese's 1967 book The Genetic Code.[19] Hans Kuhn in 1972 laid out a possible process by which the modern genetic system might have arisen from a nucleotide-based precursor, and this led Harold White in 1976 to observe that many of the cofactors essential for enzymatic function are either nucleotides or could have been derived from nucleotides. He proposed a scenario whereby the critical electrochemistry of enzymatic reactions would have necessitated retention of the specific nucleotide moieties of the original RNA-based enzymes carrying out the reactions, while the remaining structural elements of the enzymes were gradually replaced by protein, until all that remained of the original RNAs were these nucleotide cofactors, "fossils of nucleic acid enzymes".[20] The phrase "RNA World" was first used by Nobel laureate Walter Gilbert in 1986, in a commentary on how recent observations of the catalytic properties of various forms of RNA fit with this hypothesis.[21]
Properties of RNA
The properties of RNA make the idea of the RNA world hypothesis conceptually plausible, though its general acceptance as an explanation for the origin of life requires further evidence.
RNA as an enzyme
In the 1980s, RNA structures capable of self-processing were discovered,[27] with the RNA moiety of RNase P acting as its catalytic subunit.[28] These catalytic RNAs were referred to as RNA enzymes, or ribozymes, are found in today's DNA-based life and could be examples of living fossils. Ribozymes play vital roles, such as that of the ribosome. The large subunit of the ribosome includes an rRNA responsible for the peptide bond-forming peptidyl transferase activity of protein synthesis. Many other ribozyme activities exist; for example, the hammerhead ribozyme performs self-cleavage[29] and an RNA polymerase ribozyme can synthesize a short RNA strand from a primed RNA template.[30]
Among the enzymatic properties important for the beginning of life are:
- Self-replication
- The ability to self-replicate or synthesize other RNA molecules; relatively short RNA molecules that can synthesize others have been artificially produced in the lab. The shortest was 165 bases long, though it has been estimated that only part of the molecule was crucial for this function. One version, 189 bases long, had an error rate of just 1.1% per nucleotide when synthesizing an 11-nucleotide long RNA strand from primed template strands.[31] This 189-base pair ribozyme could polymerize a template of at most 14 nucleotides in length, which is too short for self-replication, but is a potential lead for further investigation. The longest primer extension performed by a ribozyme polymerase was 20 bases.[32] In 2016, researchers reported the use of in vitro evolution to improve dramatically the activity and generality of an RNA polymerase ribozyme by selecting variants that can synthesize functional RNA molecules from an RNA template.[33] Each RNA polymerase ribozyme was engineered to remain linked to its new, synthesized RNA strand; this allowed the team to isolate successful polymerases. The isolated RNA polymerases were again used for another round of evolution. After several rounds of evolution, they obtained one RNA polymerase ribozyme called 24-3 that was able to copy almost any other RNA, from small catalysts to long RNA-based enzymes. Particular RNAs were amplified up to 10,000 times, a first RNA version of the polymerase chain reaction (PCR).[33]
- Catalysis
- The ability to Bos taurus (cattle) albumin mRNA was subjected to test-tube evolution to derive a catalytic DNA (Deoxyribozyme, also called DNAzyme) with RNA-cleavage activity. After only a few weeks, a DNAzyme with significant catalytic activity had evolved.[36] In general, DNA is much more chemically inert than RNA and hence much more resistant to obtaining catalytic properties. If in vitro evolution works for DNA it will happen much more easily with RNA. In 2022, Nick Lane and coauthors showed in a computational simulation that short RNA sequences could have been capable of catalyzing CO2 fixation which supported protocell replication and growth.[37]
- Amino acid-RNA ligation
- The ability to conjugate an amino acid to the 3'-end of an RNA in order to use its chemical groups or provide a long-branched aliphatic sidechain.[38]
- Peptide bond formation
- The ability to catalyse the formation of tRNA is suggested to have evolved from RNA molecules that began to catalyze amino acid transfer.[43]
Cofactors
- Protein enzymes catalyze various chemical reactions, but over half of them incorporate cofactors to facilitate and diversify their catalytic activities.[44] Cofactors are essential in biology, as they are based largely on nucleotides rather than amino acids. Ribozymes use nucleotide cofactors to create metabolism, with two basic choices: non-covalent binding or covalent attachment. Both approaches have been demonstrated using directed evolution to reinvent RNA dupes of protein-catalyzed processes. Lorsch and Szostak [45] investigated ribozymes that could phosphorylate themselves and use ATP-γS as a substrate. However, only one of the seven classes of selected ribozymes had detectable ATP affinity, indicating that the ability to bind ATP was compromised. NAD+- dependent redox ribozymes were also evaluated.[46] The select ribozyme had a rate of enhancement of more than 107 fold and was proven to catalyze the reverse reaction - benzaldehyde reduction by NADH.[47] Since the usage of adenosine as a cofactor is prevalent in current metabolism and is likely to have been common in the RNA world, these discoveries are essential for the evolution of metabolism in the RNA world.
RNA in information storage
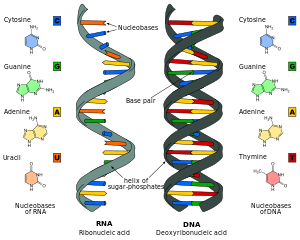
RNA is a very similar molecule to DNA, with only two significant chemical differences (the backbone of RNA uses ribose instead of deoxyribose and its nucleobases include uracil instead of thymine). The overall structure of RNA and DNA are immensely similar—one strand of DNA and one of RNA can bind to form a double helical structure. This makes the storage of information in RNA possible in a very similar way to the storage of information in DNA. However, RNA is less stable, being more prone to hydrolysis due to the presence of a hydroxyl group at the ribose 2' position.
Comparison of DNA and RNA structure
The major difference between RNA and DNA is the presence of a
RNA also uses a different set of bases than DNA—adenine, guanine, cytosine and uracil, instead of adenine, guanine, cytosine and thymine. Chemically, uracil is similar to thymine, differing only by a methyl group, and its production requires less energy.[48] In terms of base pairing, this has no effect. Adenine readily binds uracil or thymine. Uracil is, however, one product of damage to cytosine that makes RNA particularly susceptible to mutations that can replace a GC base pair with a GU (wobble) or AU base pair.
RNA is thought to have preceded DNA, because of their ordering in the biosynthetic pathways.[5] The deoxyribonucleotides used to make DNA are made from ribonucleotides, the building blocks of RNA, by removing the 2'-hydroxyl group. As a consequence, a cell must have the ability to make RNA before it can make DNA.
Limitations of information storage in RNA
The chemical properties of RNA make large RNA
RNA as a regulator
Riboswitches have been found to act as regulators of gene expression, particularly in bacteria, but also in plants and
Support and difficulties
The RNA world hypothesis is supported by RNA's ability to do all three of to store, to transmit, and to duplicate
Since there were no known chemical pathways for the abiogenic synthesis of nucleotides from pyrimidine nucleobases cytosine and uracil under prebiotic conditions, it is thought by some that nucleic acids did not contain these nucleobases seen in life's nucleic acids.[58] The nucleoside cytosine has a half-life in isolation of 19 days at 100 °C (212 °F) and 17,000 years in freezing water, which some argue is too short on the geologic time scale for accumulation.[59] Others have questioned whether ribose and other backbone sugars could be stable enough to be found in the original genetic material,[60] and have raised the issue that all ribose molecules would have had to be the same enantiomer, as any nucleotide of the wrong chirality acts as a chain terminator.[61]
Pyrimidine ribonucleosides and their respective nucleotides have been prebiotically synthesised by a sequence of reactions that by-pass free sugars and assemble in a stepwise fashion by including nitrogenous and oxygenous chemistries. In a series of publications,
The Sutherland group's 2009 paper also highlighted the possibility for the photo-sanitization of the pyrimidine-2',3'-cyclic phosphates.[23] A potential weakness of these routes is the generation of enantioenriched glyceraldehyde, or its 3-phosphate derivative (glyceraldehyde prefers to exist as its keto tautomer dihydroxyacetone).[citation needed]
On August 8, 2011, a report, based on
A study in 2001 shows that nicotinic acid and its precursor, quinolinic acid can be "produced in yields as high as 7% in a six-step nonenzymatic sequence from aspartic acid and dihydroxyacetone phosphate (DHAP). The biosynthesis of ribose phosphate could have produced DHAP and other three carbon compounds. Aspartic acid could have been available from prebiotic synthesis or from the ribozyme synthesis of pyrimidines." This supports that NAD could have originated in the RNA world.[75] RNA sequences at lengths of 30 nucleotides, 60 nucleotides, 100 nucleotides, and 140 nucleotides, were capable of catalysis of "the synthesis of three common coenzymes, CoA, NAD, and FAD, from their precursors, 4‘-phosphopantetheine, NMN, and FMN, respectively".[76]
Prebiotic RNA synthesis

Nucleotides are the fundamental molecules that combine in series to form RNA. They consist of a nitrogenous base attached to a sugar-phosphate backbone. RNA is made of long stretches of specific nucleotides arranged so that their sequence of bases carries information. The RNA world hypothesis holds that in the primordial soup (or sandwich), there existed free-floating nucleotides. These nucleotides regularly formed bonds with one another, which often broke because the change in energy was so low. However, certain sequences of base pairs have catalytic properties that lower the energy of their chain being created, enabling them to stay together for longer periods of time. As each chain grew longer, it attracted more matching nucleotides faster, causing chains to now form faster than they were breaking down.
These chains have been proposed by some as the first, primitive forms of life. In an RNA world, different sets of RNA strands would have had different replication outputs, which would have increased or decreased their frequency in the population, i.e., natural selection. As the fittest sets of RNA molecules expanded their numbers, novel catalytic properties added by mutation, which benefitted their persistence and expansion, could accumulate in the population. Such an autocatalytic set of ribozymes, capable of self-replication in about an hour, has been identified. It was produced by molecular competition (in vitro evolution) of candidate enzyme mixtures.[77]
Competition between RNA may have favored the emergence of cooperation between different RNA chains, opening the way for the formation of the first protocell. Eventually, RNA chains developed with catalytic properties that help amino acids bind together (a process called peptide-bonding). These amino acids could then assist with RNA synthesis, giving those RNA chains that could serve as ribozymes the selective advantage. The ability to catalyze one step in protein synthesis, aminoacylation of RNA, has been demonstrated in a short (five-nucleotide) segment of RNA.[78]
In March 2015, NASA scientists reported that, for the first time, complex DNA and RNA organic compounds of
In 2018, researchers at
Evolution of DNA
One of the challenges posed by the RNA world hypothesis is to discover the pathway by which an RNA-based system transitioned to one based on DNA. Geoffrey Diemer and Ken Stedman, at Portland State University in Oregon, may have found a solution. While conducting a survey of viruses in a hot acidic lake in Lassen Volcanic National Park, California, they uncovered evidence that a simple DNA virus had acquired a gene from a completely unrelated RNA-based virus. Virologist Luis Villareal of the University of California Irvine also suggests that viruses capable of converting an RNA-based gene into DNA and then incorporating it into a more complex DNA-based genome might have been common in the Virus world during the RNA to DNA transition some 4 billion years ago.[83][84] This finding bolsters the argument for the transfer of information from the RNA world to the emerging DNA world before the emergence of the last universal common ancestor. From the research, the diversity of this virus world is still with us.
Viroids
Additional evidence supporting the concept of an RNA world has resulted from research on viroids, the first representatives of a novel domain of "subviral pathogens".[85][86] Viroids infect plants, where most are pathogens, and consist of short stretches of highly complementary, circular, single-stranded and non-coding RNA without a protein coat. They are extremely small, ranging from 246 to 467 nucleobases, compared to the smallest known viruses capable of causing an infection, with genomes about 2,000 nucleobases in length.[87]
Based on their characteristic properties, in 1989 plant biologist
The characteristics of viroids highlighted as consistent with an RNA world were their small size, high guanine and cytosine content, circular structure, structural periodicity, the lack of protein-coding ability and, in some cases, ribozyme-mediated replication.
Origin of sexual reproduction
Eigen et al.[93] and Woese[94] proposed that the genomes of early protocells were composed of single-stranded RNA, and that individual genes corresponded to separate RNA segments, rather than being linked end-to-end as in present-day DNA genomes. A protocell that was haploid (one copy of each RNA gene) would be vulnerable to damage, since a single lesion in any RNA segment would be potentially lethal to the protocell (e.g., by blocking replication or inhibiting the function of an essential gene).
Vulnerability to damage could be reduced by maintaining two or more copies of each RNA segment in each protocell, i.e., by maintaining diploidy or polyploidy. Genome redundancy would allow a damaged RNA segment to be replaced by an additional replication of its homolog. However, for such a simple organism, the proportion of available resources tied up in the genetic material would be a large fraction of the total resource budget. Under limited resource conditions, the protocell reproductive rate would likely be inversely related to ploidy number. The protocell's fitness would be reduced by the costs of redundancy. Consequently, coping with damaged RNA genes while minimizing the costs of redundancy would likely have been a fundamental problem for early protocells.
A cost-benefit analysis was carried out in which the costs of maintaining redundancy were balanced against the costs of genome damage.[95] This analysis led to the conclusion that, under a wide range of circumstances, the selected strategy would be for each protocell to be haploid, but to periodically fuse with another haploid protocell to form a transient diploid. The retention of the haploid state maximizes the growth rate. The periodic fusions permit mutual reactivation of otherwise lethally damaged protocells. If at least one damage-free copy of each RNA gene is present in the transient diploid, viable progeny can be formed. For two, rather than one, viable daughter cells to be produced would require an extra replication of the intact RNA gene homologous to any RNA gene that had been damaged prior to the division of the fused protocell. The cycle of haploid reproduction, with occasional fusion to a transient diploid state, followed by splitting to the haploid state, can be considered to be the sexual cycle in its most primitive form.[95][96] In the absence of this sexual cycle, haploid protocells with damage in an essential RNA gene would simply die.
This model for the early sexual cycle is hypothetical, but it is very similar to the known sexual behavior of the segmented RNA viruses, which are among the simplest organisms known. Influenza virus, whose genome consists of 8 physically separated single-stranded RNA segments,[97] is an example of this type of virus. In segmented RNA viruses, "mating" can occur when a host cell is infected by at least two virus particles. If these viruses each contain an RNA segment with a lethal damage, multiple infection can lead to reactivation providing that at least one undamaged copy of each virus gene is present in the infected cell. This phenomenon is known as "multiplicity reactivation". Multiplicity reactivation has been reported to occur in influenza virus infections after induction of RNA damage by UV-irradiation,[98] and ionizing radiation.[99]
Further developments
Patrick Forterre has been working on a novel hypothesis, called "three viruses, three domains":[100] that viruses were instrumental in the transition from RNA to DNA and the evolution of Bacteria, Archaea, and Eukaryota. He believes the last universal common ancestor[100] was RNA-based and evolved RNA viruses. Some of the viruses evolved into DNA viruses to protect their genes from attack. Through the process of viral infection into hosts the three domains of life evolved.[100][101]
Another interesting proposal is the idea that RNA synthesis might have been driven by temperature gradients, in the process of thermosynthesis.[102] Single nucleotides have been shown to catalyze organic reactions.[103]
Alternative hypotheses
The hypothesized existence of an RNA world does not exclude a "Pre-RNA world", where a metabolic system based on a different nucleic acid is proposed to pre-date RNA. A candidate nucleic acid is peptide nucleic acid (PNA), which uses simple peptide bonds to link nucleobases.[106] PNA is more stable than RNA, but its ability to be generated under prebiological conditions has yet to be demonstrated experimentally.
Threose nucleic acid (
An alternative—or complementary—theory of RNA origin is proposed in the
The
Some of the difficulties of producing the precursors on earth are bypassed by another alternative or complementary theory for their origin, panspermia. It discusses the possibility that the earliest life on this planet was carried here from somewhere else in the galaxy, possibly on meteorites similar to the Murchison meteorite.[111] Sugar molecules, including ribose, have been found in meteorites.[112][113] Panspermia does not invalidate the concept of an RNA world, but posits that this world or its precursors originated not on Earth but rather another, probably older, planet.
The relative chemical complexity of the nucleotide and the unlikelihood of it spontaneously arising, along with the limited number of combinations possible among four base forms, as well as the need for RNA polymers of some length before seeing enzymatic activity, have led some to reject the RNA world hypothesis in favor of a metabolism-first hypothesis, where the chemistry underlying cellular function arose first, along with the ability to replicate and facilitate this metabolism.
RNA-peptide coevolution
Another proposal is that the dual-molecule system we see today, where a nucleotide-based molecule is needed to synthesize protein, and a peptide-based (protein) molecule is needed to make nucleic acid polymers, represents the original form of life.[114] This theory is called RNA-peptide coevolution,[115] or the Peptide-RNA world, and offers a possible explanation for the rapid evolution of high-quality replication in RNA (since proteins are catalysts), with the disadvantage of having to postulate the coincident formation of two complex molecules, an enzyme (from peptides) and a RNA (from nucleotides). In this Peptide-RNA World scenario, RNA would have contained the instructions for life, while peptides (simple protein enzymes) would have accelerated key chemical reactions to carry out those instructions.[116] The study leaves open the question of exactly how those primitive systems managed to replicate themselves — something neither the RNA World hypothesis nor the Peptide-RNA World theory can yet explain, unless polymerases (enzymes that rapidly assemble the RNA molecule) played a role.[116]
A research project completed in March 2015 by the Sutherland group found that a network of reactions beginning with hydrogen cyanide and
Implications
The RNA world hypothesis, if true, has important implications for the
The RNA world hypothesis places RNA at center-stage when life originated. The RNA world hypothesis is supported by the observations that ribosomes are ribozymes:
RNAs are known to play roles in other cellular catalytic processes, specifically in the targeting of enzymes to specific RNA sequences. In eukaryotes, the processing of
See also
- GADV-protein world hypothesis
- The Major Transitions in Evolution
- RNA-based evolution
- Protocell or Pre-cell, the primordial version of a cell which confined RNA and later, DNA
- First universal common ancestor (FUCA)
References
- ^ Johnson, Mark (9 March 2024). "'Monumental' experiment suggests how life on Earth may have started". The Washington Post. Archived from the original on 9 March 2024. Retrieved 10 March 2024.
- ^ PMID 23551238.
[The RNA world's existence] has broad support within the community today.
- ^ PMID 21441585.
- PMID 25803468.
- ^ PMID 20739415.
- ^ Wade, Nicholas (May 4, 2015). "Making Sense of the Chemistry That Led to Life on Earth". The New York Times. Archived from the original on July 9, 2017. Retrieved May 10, 2015.
- PMID 17897696.
The proposal that life on Earth arose from an RNA World is widely accepted.
- S2CID 203719976.
- S2CID 43793294.
- ^ Zimmer, Carl (September 25, 2014). "A Tiny Emissary from the Ancient Past". The New York Times. Archived from the original on September 27, 2014. Retrieved September 26, 2014.
- ^ S2CID 22282629.
- PMID 27375676.
- ^ Garwood RJ (2012). "Patterns In Palaeontology: The first 3 billion years of evolution". Palaeontology Online. 2 (11): 1–14. Archived from the original on June 26, 2015. Retrieved June 25, 2015.
- S2CID 4939632.
- PMID 26791312.
- ISBN 978-0-12-400450-4.
- S2CID 4144681.
- PMID 5718557.
- ^ Woese C.R. (1967). The genetic code: The molecular basis for genetic expression. p. 186. Harper & Row
- S2CID 22282629.
- S2CID 8026658.
- ^ ISBN 978-0-87969-739-6.
- ^ S2CID 4412117.
- S2CID 83662769.
- PMID 10760258.
- S2CID 23526930.
- S2CID 14787080.
- S2CID 39111511.
- S2CID 33415709.
- (PDF) from the original on 2012-02-27.
- S2CID 14174984.
- PMID 17586759.
- ^ PMID 27528667.
- PMID 9831528.
- S2CID 9734076.
- PMID 26091540.
- PMID 36350219.
- PMID 21779963.
- PMID 1604315.
- PMID 21930590.
- PMID 35137169.
- S2CID 4398830.
- PMID 10354582.
- ISSN 0044-8249.
- S2CID 40795082.
- S2CID 41081956.
- PMID 15099068.
- ^ "Uracil". Archived from the original on 2015-09-08. Retrieved 2020-07-24.
- S2CID 4283694.
- S2CID 5288515.
- PMID 16153177.
- PMID 14729327.
- PMID 15919195.
- PMID 18778966.
- PMID 16438677.
- ISBN 978-1-904455-21-9
- ^ Bell, Graham: The Basics of Selection. Springer, 1997.[page needed]
- PMID 7524147.
- PMID 9653118.
- PMID 7667262.
- S2CID 4367383.
- ^ Carole Anastasi, Michael A. Crowe, Matthew W. Powner, John D. Sutherland "Direct Assembly of Nucleoside Precursors from Two- and Three-Carbon Units Angewandte Chemie International Edition 45(37):6176–79, 2006.
- S2CID 5704391.
- from the original on 2009-05-16.
- ^ Urquhart J (13 May 2009), "Insight into RNA origins", Chemistry World, Royal Society of Chemistry, archived from the original on 4 October 2015
- PMID 21836052.
- ^ Steigerwald J (8 August 2011). "NASA Researchers: DNA Building Blocks Can Be Made in Space". NASA. Archived from the original on 23 June 2015. Retrieved 2011-08-10.
- ^ ScienceDaily Staff (9 August 2011). "DNA Building Blocks Can Be Made in Space, NASA Evidence Suggests". ScienceDaily. Archived from the original on 5 September 2011. Retrieved 2011-08-09.
- PMID 28973920.
- ^ Than, Ker (August 29, 2012). "Sugar Found In Space". National Geographic. Archived from the original on July 14, 2015. Retrieved August 31, 2012.
- AP News. Archivedfrom the original on July 14, 2015. Retrieved August 31, 2012.
- (PDF) from the original on 2015-09-24.
- ISSN 2296-987X.
- ^ "Building blocks for RNA-based life abound at center of our galaxy". EurekAlert!. 2022-07-08. Retrieved 2022-07-11.
- S2CID 25458439.
- PMID 11112541.
- PMID 19131595.
- "First Examples Of RNA That Replicates Itself Indefinitely Developed By Scripps Scientists". Medical News Today. January 12, 2009. Archived from the original on 2009-07-31.
- PMID 20176971.
- "Scientists create tiny RNA molecule with big implications for life's origins". ScienceDaily (Press release). February 24, 2010.
- ^ Marlaire R (3 March 2015). "NASA Ames Reproduces the Building Blocks of Life in Laboratory". NASA. Archived from the original on 5 March 2015. Retrieved 5 March 2015.
- ^ "New Study Identifies Possible Ancestors of RNA". 2018-09-14. Archived from the original on 2020-11-09.
{{cite web}}: CS1 maint: unfit URL (link) - PMID 29308815.
- PMID 27108699.
- ^ Holmes, Bob (2012) "First Glimpse at the birth of DNA" (New Scientist April 12, 2012)
- PMID 22515485.
- PMID 5095900.
- ^ "ARS Research Timeline – Tracking the Elusive Viroid". 2006-03-02. Archived from the original on 2007-07-06. Retrieved 2007-07-18.
- PMID 1069269.
- PMID 2480600.
- PMID 16741503.
- ^ PMID 25002087.
- ^ Zimmer, Carl (September 25, 2014). "A Tiny Emissary From the Ancient Past". The New York Times. Archived from the original on November 29, 2014. Retrieved November 22, 2014.
- PMID 27016066
- PMID 6164094.
- ISBN 978-0-521-28933-7. pp. 209-233.
- ^ PMID 6209512.
- ISBN 978-0-12-092860-6. see pgs. 293-297
- PMID 6351727.
- PMID 13687359.
- S2CID 4200194.
- ^ PMID 16505372.
- S2CID 39984425.
- PMID 16024164.
- .
- ^ Zimmer, Carl (September 12, 2013). "A Far-Flung Possibility for the Origin of Life". The New York Times. Archived from the original on July 8, 2015. Retrieved September 12, 2013.
- ^ Webb R (August 29, 2013). "Primordial broth of life was a dry Martian cup-a-soup". New Scientist. Archived from the original on April 24, 2015. Retrieved September 13, 2013.
- S2CID 4318153.
- ^ Platts SN. "The PAH World – Discotic polynuclear aromatic compounds as a mesophase scaffolding at the origin of life". Archived from the original on 2011-02-03.
- ^ Allamandola L. "Cosmic Distribution of Chemical Complexity". Archived from the original on 2014-02-27.
- ^ Atkinson, Nancy (2010-10-27). "Buckyballs Could Be Plentiful in the Universe". Universe Today. Archived from the original on 2010-10-29. Retrieved 2010-10-28.
- S2CID 33588270.
- PMID 10024233.
- ^ Steigerwald, Bill; Jones, Nancy; Furukawa, Yoshihiro (18 November 2019). "First Detection of Sugars in Meteorites Gives Clues to Origin of Life". NASA. Retrieved 18 November 2019.
- PMID 31740594.
- S2CID 5616924.
- ISBN 978-0-387-45083-4
- ^ a b "Challenging Assumptions About the Origin of Life". Astrobiology Magazine. 18 September 2013. Archived from the original on 8 May 2014. Retrieved 2014-05-07.
{{cite journal}}: CS1 maint: unfit URL (link) - ^ PMID 25803468.
- from the original on 12 August 2015. Retrieved 2015-07-26.
- PMID 29359747.
- Scripps Research Institute. November 6, 2017. Archivedfrom the original on 7 November 2017. Retrieved 7 November 2017.
- PMID 20534711.
- S2CID 27493054.
Further reading
- Attwater J, Raguram A, Morgunov AS, Gianni E, Holliger P (May 2018). "Ribozyme-catalysed RNA synthesis using triplet building blocks". eLife. 7: e35255. PMID 29759114.
- Cairns-Smith AG (1993). Genetic Takeover: And the Mineral Origins of Life. Cambridge University Press. ISBN 978-0-521-23312-5.
- Orgel LE (October 1994). "The origin of life on the earth". Scientific American. 271 (4): 76–83. PMID 7524147.
- Orgel LE (2004). "Prebiotic chemistry and the origin of the RNA world". Critical Reviews in Biochemistry and Molecular Biology. 39 (2): 99–123. S2CID 4939632.
- Woolfson A (September 2000). Life Without Genes. London: Flamingo. ISBN 978-0-00-654874-4.
- Vlassov AV, Kazakov SA, Johnston BH, Landweber LF (August 2005). "The RNA world on ice: a new scenario for the emergence of RNA information". Journal of Molecular Evolution. 61 (2): 264–273. S2CID 21096886.
- Engelhart AE, Hud NV (December 2010). "Primitive genetic polymers". Cold Spring Harbor Perspectives in Biology. 2 (12): a002196. PMID 20462999.
- Bernhardt HS (July 2012). "The RNA world hypothesis: the worst theory of the early evolution of life (except for all the others)(a)". Biology Direct. 7 (1): 23. PMID 22793875.
- Sutherland JD (April 2010). "Ribonucleotides". Cold Spring Harbor Perspectives in Biology. 2 (4): a005439. PMID 20452951.
- Camprubí E, de Leeuw JW, House CH, Raulin F, Russell MJ, Spang A, Tirumalai MR, Westall F (Dec 2019). "The Emergence of Life". Space Sci Rev. 215 (56): 56. .
External links
- "Understanding the RNA World". Exploring Life's Origins. Exploring Origins Project.
- Ferris, James P. "The Formation of the RNA World". The New York Center for Studies of the Origins of Life, Rensselaer Polytechnic Institute. Archived from the original on March 1, 2012.
- Altman, Sidney (2001). "The RNA World". NobelPrize.org. Nobel Media.
- Kuska, Robert (June 2002). "A World Apart" (PDF). HHMI Bulletin. Howard Hughes Medical Institute. pp. 14–19. Archived (PDF) from the original on 2004-05-22.
- Cech, Thomas R. (2004). "Exploring the New RNA World". NobelPrize.org. Nobel Media.
- Sutherland JD (April 2010). "Ribonucleotides". Cold Spring Harbor Perspectives in Biology. 2 (4): a005439. PMID 20452951.
- "The Origins of the RNA World". YouTube. Library of Congress. August 5, 2016.
